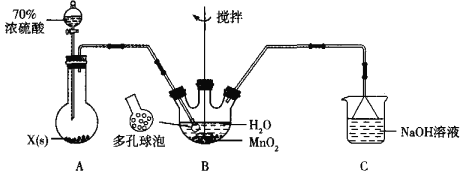
��Ŀ����
����Ŀ��ijУ�Ļ�ѧ��ȤС����������ʵ��װ��̽��������������ʣ�E��װ�г������壬��Eװ�������IJ������ܿɸ�����Ҫ�任���̡�
ʵ�鿪ʼʱ�ر�K2��K1���ӷ�Һ©��������ƿ�ڼ���Ũ���ᡣ
(1)A�з�����Ӧ�Ļ�ѧ����ʽ�ǣ�________________________________________��
(2)C�е�������______________��֤��SO2��____________�ԡ�
(3)D��������_____________________����Ӧ�����ӷ���ʽΪ____________��
(4)A�з�Ӧ��ɺر�K1����K2����E�е���ɫ���建��ע��B�У��а�ɫ����������
����E����ɫ�д̼�����ζ���壬���ķ���ʽΪ________ (�ѧʽ)
����E����ɫ��ζ���壬 �����İ�ɫ������_________(�ѧʽ)��
(5)����B����Һ����Ba(NO3)2��Һ�������а�ɫ�������ɣ��������ӷ���ʽ������ԭ��________________��
���𰸡�Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O ��ɫ��ȥ ��ԭ ���ն���SO2����ֹ��Ⱦ���� SO2+2OH-=SO32-+H2O NH3 BaSO4 3SO2+2NO3-+3Ba2++2H2O=3BaSO4��+2NO+4H+
CuSO4+SO2��+2H2O ��ɫ��ȥ ��ԭ ���ն���SO2����ֹ��Ⱦ���� SO2+2OH-=SO32-+H2O NH3 BaSO4 3SO2+2NO3-+3Ba2++2H2O=3BaSO4��+2NO+4H+
��������
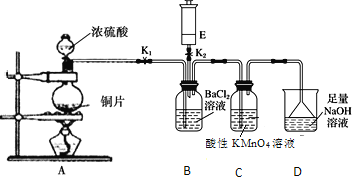
��װ��A��Cu��Ũ�����ϼ��ȷ�Ӧ����SO2���壬��SO2ͨ��Bװ�õ�BaCl2��Һ�ж��߲��ܷ�����Ӧ����SO2ͨ������KMnO4��Һ�����߷���������ԭ��Ӧ����Һ����ɫ��ȥ��SO2�Ǵ�����Ⱦ��ɸ������������������ܹ���Ӧ��������NaOH��Һ����β��������Ȼ���ŷš���װ��E������ͨ��B�У��������ɫ��������E����ɫ�д̼�����ζ�����壬���������NH3��NH3��SO2��H2O��Ӧ����(NH4)2SO3��(NH4)2SO3��BaCl2����BaSO3��ɫ��������E����ɫ��ζ�����壬���������O2��O3���ܹ����ܽ�����Һ��SO2��H2O��Ӧ����H2SO4��H2SO4��BaCl2����BaSO4��ɫ������SO2��H+��NO3-�ᷢ��������ԭ��Ӧ����SO42-���ݴ˷������
(1)A��Cu��Ũ�����ϼ��ȷ�����Ӧ����CuSO4��SO2��H2O��������Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
(2)����KMnO4��Һ����ǿ�������ԣ�SO2���л�ԭ�ԣ���������Һ�з���������ԭ��Ӧ��ʹ����KMnO4��Һ����ɫ��ȥ����˿���C�е���������ɫ��Ϊ��ɫ��֤��SO2���л�ԭ�ԣ�
(3)D��NaOH�������ն����SO2���壬��ֹ������Ⱦ����D�����������ն���SO2����ֹ��Ⱦ�������÷�Ӧ�����ӷ���ʽΪ��SO2+2OH-=SO32-+H2O��
(4)��SO2���������壬�ܹ������кͷ�Ӧ�����������Σ���E����ɫ�д̼�����ζ���壬���������NH3����SO2����Һ�з�Ӧ����(NH4)2SO3��(NH4)2SO3��BaCl2����BaSO3��ɫ������
��SO2����ǿ�Ļ�ԭ�ԣ���E����ɫ��ζ���壬���������O2��O3��O2��O3����ǿ�������ԣ��������ܽ�����Һ��SO2��H2O��Ӧ����H2SO4��H2SO4��BaCl2����BaSO4��ɫ������
(5)SO2����ˮ��Ӧ����H2SO3��ʹ��Һ�����ԣ�������������NO3-����ǿ�������ԣ����Խ�SO2��H2SO3����ΪH2SO4������SO42-��Ba2+����γ�BaSO4��ɫ�����������ӷ���ʽ��ʾΪ��3SO2+2NO3-+3Ba2++2H2O=3BaSO4��+2NO+4H+��

����Ŀ���ϳ�������Ҫ���ΪCO��H2������Ȼ��Ϊԭ�������ĺϳ����ж��ַ���������Sparg���յ�ԭ��ΪCH4(g)��CO2(g)2CO(g)��2H2(g)�����ض��¶��£���ס��ҡ��������ܱ������г�����ͬ����CH4(g)��CO2(g)���ı�������������ƽ��ʱ�����������Ũ�����±���ʾ��
ʵ���� | �����¶�/�� | ������� | ����Ũ��/(mol��L��1) | ||
CH4 | CO2 | CO | |||
�� | 300 | V1 | 0.02 | 0.02 | 0.10 |
�� | 300 | V2 | x | x | 0.05 |
�� | 350 | V1 | y | y | 0.12 |
����˵����ȷ����(����)
A.�÷�Ӧ�ڵ��������²����Է�����
B.300 ��ʱ��Ӧ��ƽ�ⳣ��Ϊ25
C.V1��V2��3��7
D.�����¶Ⱥ�����������䣬��ʼʱ��������г���0.28 mol CO��0.28 mol H2��COת����һ������2/7
����Ŀ���������ӷ���ʽ����д�����۾���������( )
ѡ�� | ���ӷ���ʽ | ���� |
A | ��2 mol Cl2ͨ�뺬1 mol FeI2����Һ�У� 2Fe2����2I����2Cl2===2Fe3����4Cl����I2 | ��ȷ��Cl2�������ɽ�Fe2����I�������� |
B | Ba(HCO3)2��Һ��������NaOH��Һ��Ӧ�� Ba2����HCO3-��OH��===BaCO3����H2O | ��ȷ����ʽ����Ӧ�������κ�ˮ |
C | ����SO2ͨ��NaClO��Һ�У� SO2��H2O��ClO��===HClO��HSO3- | ��ȷ��˵�����ԣ�H2SO3ǿ��HClO |
D | 1 mol/L��NaAlO2��Һ��2.5 mol/L��HCl��Һ�������ϣ� 2AlO2-��5H��===Al3����Al(OH)3����H2O | ��ȷ����һ����Ӧ�͵ڶ�����Ӧ���ĵ�H�������ʵ���֮��Ϊ2��3 |
A. A B. B C. C D. D