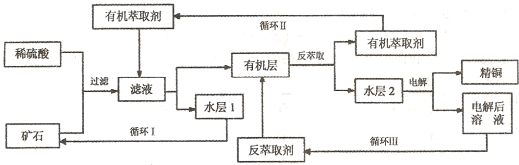
��Ŀ����
ͭ������Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
| ||
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
���ڸ÷�Ӧ�У���������12.8g Cu����ת����
| ||
0.4
0.4
mol ���ӣ�����4.48
4.48
L SO2����״�������壮��Ӧ�����Һ�м�ˮ����Һ�����Ϊ500mL��ȡ��50mL����ô50mL��Һ��CuSO4�����ʵ���Ũ��Ϊ0.4 mol/L
0.4 mol/L
��������ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ����������ˮ��
����n=
����12.8g Cu�����ʵ�������Ӧ��CuԪ����0������Ϊ+2�ۣ�ת�Ƶ������ʵ�����Cu��2����
���ݷ���ʽ�������ɶ�����������ʵ������ٸ���V=nVm�����������������
����50mL��Һ��CuSO4�����ʵ������ٸ���c=
����CuSO4�����ʵ���Ũ�ȣ�
����n=
| m |
| M |
���ݷ���ʽ�������ɶ�����������ʵ������ٸ���V=nVm�����������������
����50mL��Һ��CuSO4�����ʵ������ٸ���c=
| n |
| V |
����⣺ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ����������ˮ����Ӧ����ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
12.8g Cu�����ʵ���=
=0.2mol����Ӧ��CuԪ����0������Ϊ+2�ۣ�ת�Ƶ���d ���ʵ���=0.2mol��2=0.4mol��
�ɷ���ʽ��֪��n��SO2��=n��Cu��=0.2mol����V��SO2��=0.2mol��22.4L/mol=4.48L��
50mL��Һ��CuSO4�����ʵ���=0.2mol��
=0.02mol��CuSO4�����ʵ���Ũ��Ϊ
=0.4mol/L��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��0.4��4.48��0.4 mol/L��
| ||
12.8g Cu�����ʵ���=
| 12.8g |
| 64g/mol |
�ɷ���ʽ��֪��n��SO2��=n��Cu��=0.2mol����V��SO2��=0.2mol��22.4L/mol=4.48L��
50mL��Һ��CuSO4�����ʵ���=0.2mol��
| 50mL |
| 500mL |
| 0.02mol |
| 0.05L |
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
���������⿼����ݷ���ʽ�ļ��㡢���û�ѧ�����ļ���ȣ��Ƚϻ�����ּ�ڿ���ѧ���Ի���֪ʶ���������գ�

��ϰ��ϵ�д�
�����Ŀ
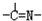 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
