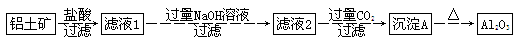��Ŀ����
��1��ʵ��������ϩ��ע����Ӧ������
| Ũ���� |
| �� |
| Ũ���� |
| �� |
��2����ϩ��������
| ���� |
| ���� |
��֪����������������ƣ������������ʣ��ٻӷ��� ������ �۱�̼�������ǿ �ܻ�ԭ�� ���ܷ���������Ӧ��
���и�ʵ���У��ֱ�������ּ�����Ӧ���ʵı�ţ�
��3����̼������Һ�м������������ų���˵���������
��4���ڼ����ƾ����м���Ũ���ᣬ���Ⱥ�ų���ʹʪ����ɫʯ����ֽ�������壬˵���������
��5�����Ҵ���Ũ�����ͺ��ȣ����ŵ�һ����ζ��˵���������
��6���ڼ�����Һ�м���������ͭ���ܿ�����Һ����ɫ��˵���������
��7��������������ͭ�м��������Һ�����ȿ����к�ɫ�������ɣ�˵���������
��2����ϩ���Ժ��Ȼ���ӳ����������飻
��3�����������ԣ�����ǿ��̼�
��4��������ʹʪ����ɫʯ����ֽ��죻
��5���������Ҵ���Ũ����������¼��Ȼ���������ζ������
��6��������Һ��������֮ͭ�䷢���кͷ�Ӧ������ͭ�κ�ˮ��
��7�������к���ȩ�����ܺ�������ͭ����Һ��Ӧ����ש��ɫ������
CH3CH2OH
| Ũ���� |
| �� |
| Ũ���� |
| �� |
��2����ϩ���Ժ��Ȼ���֮�䷢���ӳɷ�Ӧ���������飬ԭ������ʽΪ��CH2�TCH2+HCl
| ���� |
| ���� |
��3����̼������Һ�м�������ж�����̼����ų���֤�����������ԣ�����ǿ��̼�ᣬ�ʴ�Ϊ���ۣ�
��4���ڼ����ƾ����м���Ũ���ᣬ���Ⱥ�ų���ʹʪ����ɫʯ����ֽ����������ᣬ˵��������лӷ��ԣ��ʴ�Ϊ���٣�
��5���������Ҵ���Ũ����������¼��Ȼ���������ζ�������ʴ�Ϊ���ݣ�
��6��������Һ��������֮ͭ�䷢���кͷ�Ӧ������ͭ�κ�ˮ��˵������������ԣ��ʴ�Ϊ���ڣ�
��7�������к���ȩ�������л�ԭ�ԣ��ܺ�������ͭ����Һ��Ӧ����ש��ɫ�������ʴ�Ϊ���ܣ�

 �£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
�£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��1��N2H4��Nԭ�Ӻ��������ﵽ8�����ȶ��ṹ��д��N2H4�Ľṹʽ��______��
��2��ʵ���������ֹ�����ȡNH3�ķ�Ӧ��ѧ����ʽΪ______��
��3��NH3��NaClO��Ӧ�ɵõ��£�N2H4�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��4����һ����ȼ�ϵ����һ�ּ��Ի�����أ��õ�طŵ�ʱ�������ķ�ӦʽΪ______��
��5����ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO��NH2��2]��Ӧ�Ļ�ѧ����ʽΪ2NH3��g��+CO2��g��?CO��NH2��2��l��+H2O��l�����÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
| T/�� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
���ʱ��H______0�����������������=������
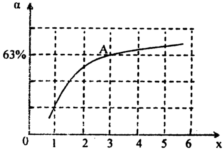
����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�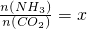 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����______��ͼ��A�㴦��NH3��ƽ��ת����Ϊ______��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����______��ͼ��A�㴦��NH3��ƽ��ת����Ϊ______��
��6���ں��º����ܱ������а��ռס��ҡ������ַ�ʽ�ֱ�Ͷ�ϣ�������Ӧ��N2��g��+3H2��g��?2NH3��g������ü�������H2��ƽ��ת����Ϊ40%��
| n��N2�� | n��H2�� | n��NH3�� | |
| �� | 1mol | 3mol | 0mol |
| �� | 0.5mol | 1.5mol | 1mol |
| �� | 0mol | 0mol | 4mol |
���ж��������з�Ӧ���еķ�����______�������������ƶ���
�ڴ�ƽ��ʱ���ס��ҡ�����������NH3�����������С˳��Ϊ______��
 ��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
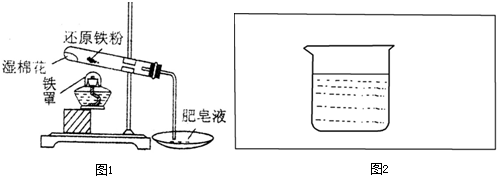
 ����ʾ�������һ��ԭ���װ�ã�����֤�е����������������ͼ2�����ڲ���ȫװ�ü�ͼ��Ҫ���ע��������������Ϻ͵������Һ���ƣ���
����ʾ�������һ��ԭ���װ�ã�����֤�е����������������ͼ2�����ڲ���ȫװ�ü�ͼ��Ҫ���ע��������������Ϻ͵������Һ���ƣ���