��Ŀ����
����Ŀ��(1)д���뷽��ʽ��NaHCO3__________________��H2CO3______________��
(2)�����������Ǵ�����������3����ԭ�ӵ�ԭ�ӽṹʾ��ͼ________________�������ӵĽṹʾ��ͼ____________��
(3)����3��С���е����ʾ��ɺ˵����Ϊ1��10��Ԫ����ɣ���Ҫ����д��ѧʽ��
�������ֱ�Ϊ4����6�����ӵ�ԭ���γɵĻ�������_________��__________��
����5��ԭ����ɵĵ�������Ϊ10�ķ�����___________��
���������������6�����ӵ�ԭ�ӽ�ϳɵķ�����____________��
���𰸡�NaHCO3=Na++HCO3- H2CO3![]() H++HCO3-��HCO3-
H++HCO3-��HCO3-![]() H++CO32-
H++CO32- 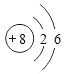
 CO CO2 CH4 O3
CO CO2 CH4 O3
��������
(1) NaHCO3���Σ�����ǿ����ʣ��������Na+��HCO3-��H2CO3�Ƕ�Ԫ���ᣬ�ֲ����룬���ڵ���ƽ�⣻
(2)��ȷ�������������Ǵ�����������3����ԭ����Oԭ�ӣ�����ԭ�Ӻ�������Ų�������дԭ�ӽṹʾ��ͼ��Sԭ�ӻ��2�������γ�S2-���ݴ���д���ӽṹʾ��ͼ��
(3)��ȷ����Ӧ��Ԫ�أ�Ȼ�����Ҫ����д��ѧʽ��
(1) NaHCO3���Σ�����ǿ����ʣ�����Һ�е������Na+��HCO3-�����뷽��ʽΪ��NaHCO3=Na++HCO3-��H2CO3�Ƕ�Ԫ���ᣬ���ڵ���ƽ�⣬������̷ֲ����룬��һ������ΪH2CO3![]() H++HCO3-���ڶ������뷽��ʽΪHCO3-
H++HCO3-���ڶ������뷽��ʽΪHCO3-![]() H++CO32-��
H++CO32-��
(2)�����������Ǵ�����������3����ԭ����Oԭ�ӣ�O��8��Ԫ�أ�ԭ�Ӻ�������Ų���2��6������Oԭ�ӽṹʾ��ͼΪ��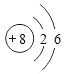 ��S��16��Ԫ�أ�Sԭ�ӻ��2�������γ�S2-����S2-���ӽṹʾ��ͼ��
��S��16��Ԫ�أ�Sԭ�ӻ��2�������γ�S2-����S2-���ӽṹʾ��ͼ�� ��
��
(3)�˵����Ϊ1~10��Ԫ���У��������ֱ�Ϊ4����6�����ӵ�ԭ�ӷֱ���Cԭ�ӡ�Oԭ�ӣ�������Ԫ���γɵĻ�������CO��CO2��
����5��ԭ����ɵĵ�������Ϊ10�ķ�����CH4��
���������6�����ӵ�ԭ����Oԭ�ӣ��������������6�����ӵ�ԭ�ӽ�ϳɵķ�����O3��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�����Ŀ��ʵ�������Ʊ����ȱ�����ķ�Ӧ�Լ�װ����ͼ��ʾ��
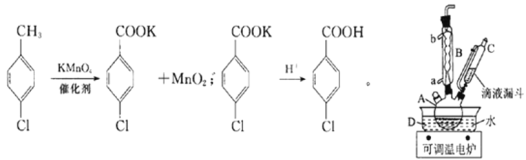
�����£����ʵ��й����ݺ����������ʾ��
�۵�/�� | �е�/�� | �ܶ�/g��cm��3 | ��ɫ | ˮ���� | |
���ȼױ� | 7.5 | 162 | 1.07 | ��ɫ | ���� |
���ȱ����� | 243 | 275 | 1.54 | ��ɫ | �� |
���ȱ������ | �����ε�ͨ�ԣ����ڿ������� | ||||
ʵ�鲽�裺�ڹ��Ϊ250mL������A�м���һ�����Ĵ���������KMnO4��100mLˮ����װ��װ�ã��ڵ�Һ©���м���6.00mL���ȼױ������¶�Ϊ93������ʱ����ε�����ȼױ��������¶���93�����ң���Ӧ2h�����ˣ�����������ˮϴ�ӣ�ʹϴ��Һ����Һ�ϲ�������ϡ�����ữ������Ũ������ȴ��Ȼ����ˣ�����������ˮ����ϴ�ӣ�����������������
��ش��������⣺
(1)����A������Ϊ______________________��
(2)����B�������ܣ�������Ҫ�����ǣ�________________��ʵ������У���ȴˮ��________�ڡ�
(3)ʵ����������ι��ˡ�ϴ�Ӳ�������һ�ι��˵������ɷ�Ϊ___________(�ѧʽ)��ϴ�Ӹ���������ˮ��Ŀ����_________________________________���ڶ��ι��˺�ϴ����������ˮ��Ŀ����______________________��
(4)���ˡ�ϴ�Ӳ��������õ���������___________(��ѡ����ĸ)��
a.�ձ� b.��Һ©�� c.��ƿ d.������
(5)��һ�ι��˺����Һ�м������ᣬ���ֵ�������___________��
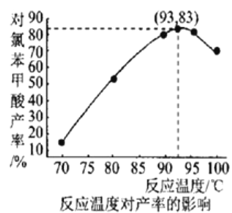
(6)��ͼ���¶ȶԶ��ȱ�������ʵ�Ӱ���ϵ������������õ��Ķ��ȱ����������Ϊ___________(����С�������λ)��