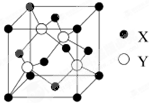
��Ŀ����
3������ͼʾ���
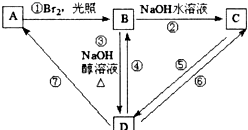
��1��������A�к��������ŵ�������ȩ�����Ȼ���
��2��1mol A��2mol H2��Ӧ����1mol E���䷴Ӧ����ʽ��OHCCH=CHCOOH+2H2$\stackrel{һ��������}{��}$HOCH2CH2CH2COOH��
��3��B��������������Br2��Ӧ�õ�D��D�Ľṹ��ʽ��HOOCCHBrCHBrCOOH��д��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽHOOCCHBrCHBrCOOH+4NaOH$\stackrel{��}{��}$NaOOCCH��OH��CH��OH��COONa+2H2O+2NaBr
��4��F�Ľṹ��ʽ��
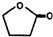 ��
��
���� A�ܺ�̼�����Ʒ�Ӧ��˵��A�к����Ȼ���A�ܺ�������Һ����������Ӧ��˵��A�к���ȩ����F����Ԫ��״�����F�����Ͷ�=$\frac{4��2+2-6}{2}$=1��1mol A��2mol H2��Ӧ����1mol E����A������һ��̼̼˫������A�����ӳɷ�Ӧ���д��ǻ���A���������Ȼ�������E����������Ӧ����F��
A����������ӦȻ���ữ����B��B�к��������Ȼ���B���巢���ӳɷ�Ӧ����D��D��̼��û��֧������D�ṹ��ʽΪHOOCCHBrCHBrCOOH��BΪHOOCCH=CHCOOH��AΪOHCCH=CHCOOH��CΪOHCCH=CHCOONa��EΪHOCH2CH2CH2COOH��FΪ ���ݴ˷������
���ݴ˷������
��� �⣺A�ܺ�̼�����Ʒ�Ӧ��˵��A�к����Ȼ���A�ܺ�������Һ����������Ӧ��˵��A�к���ȩ����F����Ԫ��״�����F�����Ͷ�=$\frac{4��2+2-6}{2}$=1��1mol A��2mol H2��Ӧ����1mol E����A������һ��̼̼˫������A�����ӳɷ�Ӧ���д��ǻ���A���������Ȼ�������E����������Ӧ����F��
A����������ӦȻ���ữ����B��B�к��������Ȼ���B���巢���ӳɷ�Ӧ����D��D��̼��û��֧������D�ṹ��ʽΪHOOCCHBrCHBrCOOH��BΪHOOCCH=CHCOOH��AΪOHCCH=CHCOOH��CΪOHCCH=CHCOONa��EΪHOCH2CH2CH2COOH��FΪ ��
��
��1��AΪOHCCH=CHCOOH������������������ȩ�����Ȼ����ʴ�Ϊ��ȩ�����Ȼ���
��2��1mol A��2mol H2��Ӧ����1mol E��AΪOHCCH=CHCOOH��EΪHOCH2CH2CH2COOH����Ӧ����ʽΪOHCCH=CHCOOH+2H2$\stackrel{һ��������}{��}$HOCH2CH2CH2COOH��
�ʴ�Ϊ��OHCCH=CHCOOH+2H2$\stackrel{һ��������}{��}$HOCH2CH2CH2COOH��
��3��ͨ�����Ϸ���֪��D�ṹ��ʽΪHOOCCHBrCHBrCOOH��D���������Ƶ�ˮ��Һ����ȡ����Ӧ����HOOCCH��OH��CH��OH��COOH����Ӧ����ʽΪHOOCCHBrCHBrCOOH+4NaOH$\stackrel{��}{��}$NaOOCCH��OH��CH��OH��COONa+2H2O+2NaBr��
�ʴ�Ϊ��HOOCCHBrCHBrCOOH��HOOCCHBrCHBrCOOH+4NaOH$\stackrel{��}{��}$NaOOCCH��OH��CH��OH��COONa+2H2O+2NaBr��
��4��ͨ�����Ϸ���֪��F�ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ����ؿ���ѧ�������ƶ����������ݷ�Ӧ������ijЩ���ʽṹ�����ƶϣ���ȷ�ƶϸ����ʽṹ��ʽ�ǽⱾ��ؼ�����Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��H��0��ʾ���ȷ�Ӧ����H��0��ʾ���ȷ�Ӧ | |
| B�� | �����Ӧ�������е�������С�������������е��������������ķ�Ӧ�Ƿ��ȷ�Ӧ | |
| C�� | ��ϡ��Һ��1mol���1mol����ȫ��Ӧ���ų��������������к��� | |
| D�� | 1 mol H2��0.5 mol O2��Ӧ�ų����Ⱦ���H2��ȼ���� |
| A�� | 22.4LCl2���NA��Cl2���� | |
| B�� | 1L 0.1mol��L-1Na2SO4��Һ����0.1NA��Na+ | |
| C�� | ��1L1mol��L-1��NaCl��Һ��ȡ��10mL�������ʵ���Ũ��Ϊ0.01mol��L-1 | |
| D�� | 1molCa���Ca2+ʱʧȥ�ĵ�����Ϊ2NA |
| ��ѧ����ʽ | K ��t1�� | K ��t2�� |
| F2+H2?2HF | 1.8��1036 | 1.9��1032 |
| Cl2+H2?2HCl | 9.7��1012 | 4.2��1011 |
| Br2+H2?2HBr | 5.6��107 | 9.3��106 |
| I2+H2?2HI | 43 | 34 |
��2��HX�ĵ���ʽ��
 ��
����3�����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ��HX���ۼ��ļ�����ǿ������˳����HF��HCl��HBr��HI��
��4��������K�ı仯�������ƶϳ�������±��ԭ�Ӻ˵���������ӣ�ad��
a������ͬ�����£�ƽ��ʱX2��ת��������
b��X2��H2��Ӧ�ľ��ҳ̶�����
c��HX�Ļ�ԭ������
d��HX���ȶ���������
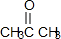 +2H2O��
+2H2O�� ��
��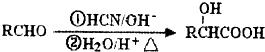
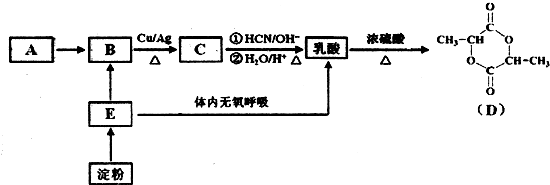
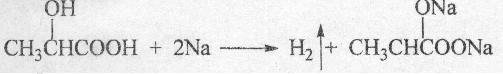 ��
�� ��
��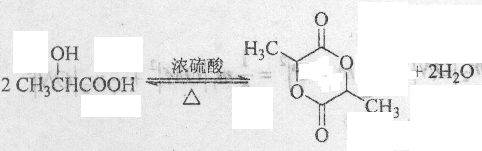 ��
�� ��
��