��Ŀ����
(8��)��ʹ������к͵ζ����ⶨ���۰״�������(g/100 mL)��
��.ʵ�鲽�裺
(1)��ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100 mL________(���� ������)�ж��ݣ�ҡ�ȼ��ô���״���Һ��
(2)����ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2��________��ָʾ����
(3)��ȡʢװ0.100 0 mol/L NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________ mL��
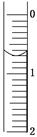
(4)�ζ�����____________________ ________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��.ʵ���¼��
|
���������ζ����� ʵ������(mL) |
1 |
2 |
3 |
4 |
|
V(��Ʒ) |
20.00 |
20.00 |
20.00 |
20.00 |
|
V(NaOH)(����) |
15.95 |
15.00 |
15.05 |
14.95 |
��.ʵ�鴦����
(1)ijͬѧ�ڴ�������ʱ����ã�
ƽ�����ĵ�NaOH��Һ�����V��(15.95��15.00��15.05��14.95)/4 mL��15.24 mL��
ָ�����ļ���IJ�����֮����____________ _ _��
(2)�ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����(��д���)________��
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
��.(1)����ƿ��(2)��̪��(3)0.70��(4)��Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫ
��.(1)��1�εζ�������Դ����쳣ֵ��Ӧ��ȥ��0.75��4.5��(2)c��(3)ab
��������
��������������к͵ζ�ԭ����ȷ������������ҩƷ��ʵ�鲽�裬��עÿ������Ҫ�켰���塣
��(1)����һ��Ҫ������ƿ�н��У�����ת�Ƶ�100 mL����ƿ�ж��ݡ�
(2)ǿ��������ĵζ������ɵ���ǿ�������Σ��Լ��ԣ����ѡ���Է�Χ�ڱ�ɫ��ָʾ�����˴�ָʾ���Ƿ�̪��
(3)ע��������ϵ������ε������˴�Ϊ0.70mL��
(4)����Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫʱ��ֹͣ�ζ���
��. (1)�����Ե�һ�����ݱ�������������ƫ����ѡ�ã�Ҫ��ȥ��
��2����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ�����±�ҺŨ��ƫ�ͣ�ʹ��ʱ���ƫ�����´���ҺŨ��ƫ��ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ���ᵼ�²ⶨ�ı�Һ���ƫ����Һ��Ũ��ƫ����ƿ�м������״���Һ���ټ�����ˮ������Ӱ��������ƿ�ڵζ�ʱ����ҡ����������Һ�彦����ʹ�õı�Һ���٣������ҺŨ��ƫС��
���㣺������Һ�����Ƽ�����к͵ζ���
����������ƽ��ֵʱ��һ��Ҫע�����ϴ������Ҫ��ȥ�������ѶȲ����ۺϡ�

(8��)��ʹ������к͵ζ����ⶨ���۰״�������(g/100 mL)��
��.ʵ�鲽�裺
(1)��ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100 mL________(���� ������)�ж��ݣ�ҡ�ȼ��ô���״���Һ��
(2)����ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2��________��ָʾ����
(3)��ȡʢװ0.100 0 mol/L NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________ mL��
(4)�ζ�����____________________ ________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��.ʵ���¼��
| ���������ζ����� ʵ������(mL) | 1 | 2 | 3 | 4 |
| V(��Ʒ) | 20.00 | 20.00 | 20.00 | 20.00 |
| V(NaOH)(����) | 15.95 | 15.00 | 15.05 | 14.95 |
��.ʵ�鴦����
(1)ijͬѧ�ڴ�������ʱ����ã�
ƽ�����ĵ�NaOH��Һ�����V��(15.95��15.00��15.05��14.95)/4 mL��15.24 mL��
ָ�����ļ���IJ�����֮����____________ _ _��
(2)�ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����(��д���)________��
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��