��Ŀ����
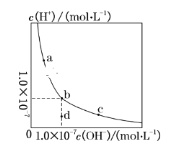
����Ŀ����֪25 ��ʱ0.1 mol��L��1������Һ��pHԼΪ3�������м�������ƾ��壬�Ⱦ����ܽ������Һ��pH�����������������ֲ�ͬ�Ľ��ͣ���ͬѧ��Ϊ������ˮ��ʼ��ԣ�������c(OH��)�������Һ��pH������ͬѧ��Ϊ����������ˮ�����������������ӣ������˴���ĵ��룬ʹc(H��)��С�������Һ��pH����
(1)�������ֽ�����________(����������������)��ȷ��
(2)Ϊ����֤�������ֽ�����ȷ������������ʵ�飺��0.1 mol��L��1�Ĵ�����Һ�м����������������е�________(��д�����ĸ)��Ȼ��ⶨ��Һ��pH��
A������CH3COOK | B������CH3COONH4 |
C������NH3 | D������NaHCO3 |
(3)��________(����������������)�Ľ�����ȷ����Һ��pHӦ________(����������������С������������)(��֪25 �� ʱ��CH3COONH4��Һ������)��
(4)�����½�0.010 mol CH3COONa��0.004 mol HCl����ˮ�����Ƴ�0.5 L�����Һ���жϣ�
����Һ�й���________�����ӡ�
����Һ�����������ӵ����ʵ����ĺ�һ������0.010 mol��������________��________��
����Һ��n(CH3COO��)��n(OH��)��n(H��)��________mol��
���𰸡��� B �� ���� 7 CH3COOH CH3COO�� 0.006
��������
(1)��������֪����Һ�д�������ƽ�⣺CH3COOH![]() CH3COO����H����CH3COO����H2O
CH3COO����H����CH3COO����H2O![]() CH3COOH��OH����������Һ�м�������ƾ��壬��Һ��pH��Ȼ����һ��С��7�����CH3COOH�ĵ���ƽ��ռ������λ����ͬѧ�Ľ�����ȷ��
CH3COOH��OH����������Һ�м�������ƾ��壬��Һ��pH��Ȼ����һ��С��7�����CH3COOH�ĵ���ƽ��ռ������λ����ͬѧ�Ľ�����ȷ��
(2)��Ϊ����֤���ֽ���������ȷ���͵�ѡ��һ�ֺ���CH3COO����ˮ�ⲻ�Լ��Ե�������ʵ�飬��ֻ����CH3COONH4��
(3) ������ȷ�����������ˮ�����������������ӣ������˴���ĵ��룬ʹc��H+����С����ҺpH����
(4)�ٻ����Һ�д��ڣ�CH3COONa��HCl=NaCl��CH3COOH��NaCl===Na����Cl����CH3COOH![]() CH3COO����H����H2O
CH3COO����H����H2O![]() H����OH������ˣ���Һ�д���Na����Cl����CH3COOH��CH3COO����H2O��H����OH����7�����ӣ�
H����OH������ˣ���Һ�д���Na����Cl����CH3COOH��CH3COO����H2O��H����OH����7�����ӣ�
�ڸ��������غ㣬n(CH3COOH)��n(CH3COO��)��n(Na��)��0.010 mol��
�۸��ݵ���غ㣬n(CH3COO��)��n(OH��)��n(Cl��)��n(Na��)��n(H��)����ˣ�n(CH3COO��)��n(OH��)��n(H��)��n(Na��)��n(Cl��)��0.010 mol��0.004 mol��0.006 mol��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�� CO2����Դ������һֱ�ǻ�ѧ���ǹ�ע����Ҫ���⣬�п�Ժ������ѧ�����о��������һ�����Ͷ�ܸ��ϴ������ɹ���ʵ����CO2ֱ�Ӽ�����ȡ������ֵ���ͣ�![]() (��Ӧ��)�����о��ɹ�������Ϊ��CO2��ת�������ͻ���Խ�չ����
(��Ӧ��)�����о��ɹ�������Ϊ��CO2��ת�������ͻ���Խ�չ����
(1)��֪������ȼ����Ϊ![]() ����Ҫ����
����Ҫ����![]() ��ȼ������a��ֵ������Ҫ֪��һ����Ӧ��
��ȼ������a��ֵ������Ҫ֪��һ����Ӧ��![]() ���÷�Ӧ��________________________________����Ӧ����һ�������¾����Է��ԣ���a_______________0(����>������<��)��
���÷�Ӧ��________________________________����Ӧ����һ�������¾����Է��ԣ���a_______________0(����>������<��)��
(2)��ij�ܱ������а�һ��Ͷ�ϱȳ���![]() ��
��![]() ����������ʹ�䷢����Ӧ��
����������ʹ�䷢����Ӧ��![]() �����
�����![]() ��ƽ��ת�������¶ȡ�ѹǿ֮��Ĺ�ϵ��ͼ1��ʾ��
��ƽ��ת�������¶ȡ�ѹǿ֮��Ĺ�ϵ��ͼ1��ʾ��
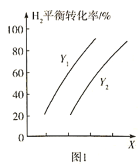
��X��ʾ______________��![]() ___________
___________![]() (����>������<��)�������
(����>������<��)�������![]() ��ƽ��ת���ʲ���ߵ�λʱ����
��ƽ��ת���ʲ���ߵ�λʱ����![]() �IJ������ɲ�ȡ�Ĵ�ʩ��______________________(������)��
�IJ������ɲ�ȡ�Ĵ�ʩ��______________________(������)��
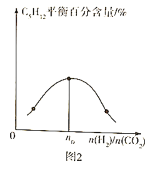
(3)����һ���¶ȡ�����������ͬͶ�ϱ�![]() ����Ӧ��ͨ�뵽ij�ܱ������У����ƽ��ʱ
����Ӧ��ͨ�뵽ij�ܱ������У����ƽ��ʱ![]() �İٷֺ�����Ͷ�ϱ�֮��Ĺ�ϵ��ͼ2��ʾ����
�İٷֺ�����Ͷ�ϱ�֮��Ĺ�ϵ��ͼ2��ʾ����![]() ____________��
____________��
(4)����-��˫���������������£�CH3OH��CO2��H2�ɸ�Ч��ת��Ϊ���ᣬ��Ӧ����ʽΪ![]() ��һ���¶��£���ij����������ͨ������ʵ���������ԭ�����������ϵ�е���ѹǿ��ʱ��Ĺ�ϵ���±���ʾ��
��һ���¶��£���ij����������ͨ������ʵ���������ԭ�����������ϵ�е���ѹǿ��ʱ��Ĺ�ϵ���±���ʾ��
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
p/kPa | 3 | 2.7 | 2.5 | 2.35 | 2.26 | 2.2 | 2.2 |
��Ӧ��ʼ���ﵽƽ��Ĺ����У�![]() ______________
______________![]() ______________
______________![]() ��
��
(5)̼�������ķ�չҲ������CO2����ԴӦ�÷���õ�������á������£���ij����NaOH��Һ�������е�CO2������Һ��pH=10���������Һ��![]() ����
����![]() _____________
_____________![]() ��
��
����Ŀ����100��ʱ����0.200 mol�������������������2 L��յ��ܱ������У�ÿ��һ����ʱ��Ը������ڵ����ʽ��з������õ����±���
| 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4) | 0.100 | c1 | 0.050 | c3 | a | b |
c(NO2) | 0.000 | 0.060 | c2 | 0.120 | 0.120 | 0.120 |
����գ�
(1)�÷�Ӧ�Ļ�ѧ����ʽΪN2O4![]() 2NO2�����ﵽƽ��ʱ��������������ת����Ϊ__________%������c2________c3��a______b(�>������<������)��
2NO2�����ﵽƽ��ʱ��������������ת����Ϊ__________%������c2________c3��a______b(�>������<������)��
(2) 20 sʱ������������Ũ��c1��________mol/L����0 s��20 sʱ����ڣ�������������ƽ����Ӧ����Ϊ________mol/(L��s)��
(3)������ͬ���������������������Ƕ����������壬Ҫ�ﵽ����ͬ����ƽ��״̬��������������ʼŨ����________mol/L��