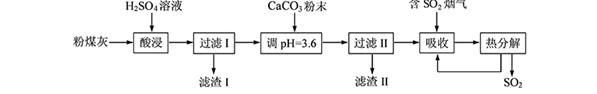
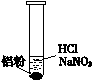
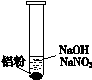
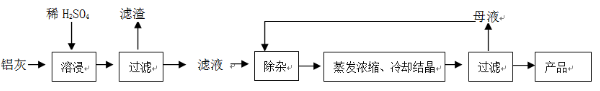
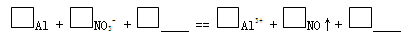��Ŀ����
������16�֣���ҵ���ð���ʯ�Ʊ��ߴ�����þ�Ĺ����������£�
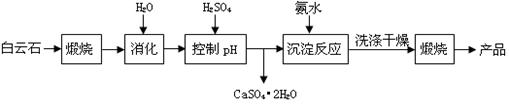
��֪��.����ʯ��Ҫ�ɷֿɱ�ʾΪ��CaO 32.50%��MgO 20.58%��Fe2O3 2.18%��SiO2 0.96%������ 43.78%��
��1��Ϊ����߰���ʯ������Ч�������Բ�ȡ�Ĵ�ʩ�ǽ���ʯ ������ʵ���������հ���ʯ����Ҫ���������ƾ��ơ����ż����⣬����Ҫ ������ţ���
A�������� B������ C�������� D��ʯ����
��2������H2SO4����pHʱ���յ�pH�Բ�Ʒ��Ӱ����ͼ8��ʾ������ͼʾ�ɵõ��Ľ��ۼ�ԭ���ǣ�
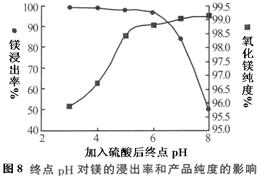
��pH���ᵼ��___________________________�½�����ԭ����_______________________
��pH���ͻ�����____________________________ ����ԭ�������__________(�����)
A.Fe2O3����H2SO4����ʹ��Ʒ�������� B.SiO2����H2SO4����ʹ��Ʒ��������
C.���Թ�ǿ���γɿ��ܵ�Ca(HSO4)2������ʹ��Ʒ���к��Ƶ�����
��3����֪MgSO4��CaSO4���ܽ�����±���
�����ϱ����ݣ���Ҫ˵������CaSO4.2H2O�IJ��������� �� ��
��4��д��������Ӧ�е����ӷ���ʽ�� ��
��5�������������л��ɵõ���һ�ָ���Ʒ��_______________��
��6����֪���ָʾ�����������ɫ��pH��Χ�����ʾ��25��ʱ��
��Mg(OH)2�ı�����Һ�еμ�2�ΰ������ָʾ������Һ�����ֵ�
��ɫΪ ��25��ʱ��Mg(OH)2���ܶȻ�Ksp=5.6��10-12����

��֪��.����ʯ��Ҫ�ɷֿɱ�ʾΪ��CaO 32.50%��MgO 20.58%��Fe2O3 2.18%��SiO2 0.96%������ 43.78%��
��1��Ϊ����߰���ʯ������Ч�������Բ�ȡ�Ĵ�ʩ�ǽ���ʯ ������ʵ���������հ���ʯ����Ҫ���������ƾ��ơ����ż����⣬����Ҫ ������ţ���
A�������� B������ C�������� D��ʯ����
��2������H2SO4����pHʱ���յ�pH�Բ�Ʒ��Ӱ����ͼ8��ʾ������ͼʾ�ɵõ��Ľ��ۼ�ԭ���ǣ�

��pH���ᵼ��___________________________�½�����ԭ����_______________________
��pH���ͻ�����____________________________ ����ԭ�������__________(�����)
A.Fe2O3����H2SO4����ʹ��Ʒ�������� B.SiO2����H2SO4����ʹ��Ʒ��������
C.���Թ�ǿ���γɿ��ܵ�Ca(HSO4)2������ʹ��Ʒ���к��Ƶ�����
��3����֪MgSO4��CaSO4���ܽ�����±���
| �¶ȣ��棩 | 40 | 50 | 60 | 70 |
| MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
| CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
��4��д��������Ӧ�е����ӷ���ʽ�� ��
��5�������������л��ɵõ���һ�ָ���Ʒ��_______________��
��6����֪���ָʾ�����������ɫ��pH��Χ�����ʾ��25��ʱ��
��Mg(OH)2�ı�����Һ�еμ�2�ΰ������ָʾ������Һ�����ֵ�
��ɫΪ ��25��ʱ��Mg(OH)2���ܶȻ�Ksp=5.6��10-12����
| pH | < 8.0 | 8.0 ~ 9.6 | > 9.6 |
| ��ɫ | ��ɫ | ��ɫ | ��ɫ |
32. �𰸣�16�֣�
��1������ ��2�֣�BC ��2�֣�
��2����þ�����ʽ��ͣ�1�֣�Mg(OH)2��MgOδ��ȫ�ܽ⣨1�֣�
�ڲ�Ʒ�����½���1�֣�AC ��1�֣�
��3�����½ᾧ�����ȹ��� ��2�֣���1�֣���
��4��Mg2+ +2NH3.H2O = Mg(OH)2��+ 2NH4+��2�֣�
��5��(NH4)2SO4��2�֣�
��6����ɫ ��2�֣�
��1������ ��2�֣�BC ��2�֣�
��2����þ�����ʽ��ͣ�1�֣�Mg(OH)2��MgOδ��ȫ�ܽ⣨1�֣�
�ڲ�Ʒ�����½���1�֣�AC ��1�֣�
��3�����½ᾧ�����ȹ��� ��2�֣���1�֣���
��4��Mg2+ +2NH3.H2O = Mg(OH)2��+ 2NH4+��2�֣�
��5��(NH4)2SO4��2�֣�
��6����ɫ ��2�֣�
��������� ��1����߿�ʯ����Ч���Ĵ�ʩͨ���ǶԿ�ʯ���з��鴦����������Ӧ�ĽӴ�������Ӷ��ӿ췴Ӧ���ʣ�ʹ���ո��ӳ�֡�����ʵ�������հ���ʯ��������Ҫ������װ���оƾ��ơ����żܡ��������������ᡢ��ǯ�ȣ����Դ˴�����ѡBC��
��2����ͼ�������������ߣ�������ʾ�����ϴ�ڵ����þ���������ߣ����������ź������յ�pH�������ֳ����½������ƣ��ҵ�pH>6ʱ���������½���Խ��Խ�죻�������ϴ�С�����������þ�Ĵ��ȣ��ô������ź������յ�pH��������ֳ���������ƣ����ǽϲ�ͬ���ǣ���ʼ����Ѹ�٣���pH>6ʱ������þ�Ĵ����������ԡ����Ԣ�pH���ᵼ��þ�Ľ����ʽ��ͣ�ԭ���Ǽ������ܽ�ʱ����pH���ߣ��������MgO������ȫ�ܽ⡣
��pH���ͻ������Ƶõ�����þ�Ĵ���ƫ�ͣ�ԭ���ǰ���ʯ�е�����������Fe2O3��CaO�����������ᣬ�������ɿ��ܵ����ʣ�����AC���п��ܣ�ѡAC��
��3���ɱ������ݿ�֪������þ������ˮ���ܽ�����¶����߶����ߣ��������������ˮ�������¶������ܽ�Ȼ������䣬�������ö����ܽ���ϵIJ��죬��Ҫ��������ƾ����������Բ�ȡ�Ĵ�ʩ�����½ᾧ�����ȹ��ˡ�
��4�����������з�Ӧ���е���ҪΪþ���ӣ����Լ��백ˮ����ˮ��þ���ӽ�����ɳ���������þ���������ӷ���ʽ��Mg2+ +2NH3.H2O = Mg(OH)2��+ 2NH4+ ��ע�ⰱˮ���ܲ�
��5����������������Mg(OH)2 ����Һ��ʣ��NH4+ ��SO4 2-�����Կ������ô�ʣ����Һ�Ƶø���Ʒ(NH4)2SO4 �������������ʡ�
��6�����͵�������þ��Һ����Mg(OH)2 ��s��= Mg2+��aq�� + 2 OH-��aq���ܽ�ƽ�⣬2c��Mg2+��= c��OH -�������ܶȻ�Ksp= c��Mg2+��c2��OH -��=5.6��10-12 ���ɴ˿��Եó�c��OH -��=3.6��10-4 ������pH>9.6�����Լ���������ָʾ������Һ����ɫ��

��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ