��Ŀ����
������������һ������Ư�ס����������㷺Ӧ����ũҵ��ҽҩ�����û����������õ�Ũ�ȵ�˫��ˮ�ͱ���������Һ��һ�������¿��Ժϳɹ��������أ���Ӧ�ķ���ʽΪ��
CO(NH2)2+H2O2 CO(NH)2��H2O2�����������صIJ����������£�
CO(NH)2��H2O2�����������صIJ����������£�
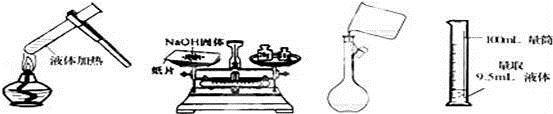
�ϳɹ��������ص����̼���Ӧװ��ͼ���£�


��ش��������⣺
��1������X��������____________������������ȴˮ��____________���a����b�����ڽ��롣
��2����Ӧ���ļ��ȷ�ʽ��_______________��
��3���������Ƿ�������ʲ���___________����ǡ�����ԭ����_______________��
��4������� ���ü�ѹ������ԭ����___________��
��5������ѡ���У����ʺ���Ϊ����� ��ϴ��Һ��_______��
a����ˮ b����ˮ c������NaCl��Һ d���ƾ���ˮ�Ļ��Һ
��6��ȷ��ȡ0.5000g��Ʒ��250mL��ƿ�У�����������ˮ�ܽ⣬�ټ�1mL 6mol/L H2SO4����0.1000 mol/L KMnO4����Һ�ζ����յ�ʱ����20.00mL��������KMnO4��Һ����Ӧ����
�� �ζ��յ��������______________��
�� ���Ʒ��CO(NH)2��H2O2����������Ϊ______________��
�� ���ζ�ǰ���ӣ��ζ����ӣ����õĹ��������غ���_________���ƫ�ߡ�����ƫ�͡����䡱����
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�