��Ŀ����
��10�֣���ʵ���������������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʣ��̶�װ����ȥ����ͼ�Т�Ϊ��ȡ������װ�ã����Թ���װ��15mL30%KOH��Һ����������ˮ�У���ȡ����أ����Թ���װ��15 m L 8%NaOH��Һ�������ڱ�ˮ�У�����װ����ɫʯ����Һ��
L 8%NaOH��Һ�������ڱ�ˮ�У�����װ����ɫʯ����Һ��

��1�����Թ�����ȡ�������ƵĻ�ѧ����ʽΪ
��2��ʵ���пɹ۲쵽���Թ�����Һ����ɫ�������±仯����д����
��3����ʵ����һ�����ԵIJ���֮����Ӧ��θĽ��� 
 L 8%NaOH��Һ�������ڱ�ˮ�У�����װ����ɫʯ����Һ��
L 8%NaOH��Һ�������ڱ�ˮ�У�����װ����ɫʯ����Һ��
��1�����Թ�����ȡ�������ƵĻ�ѧ����ʽΪ
��2��ʵ���пɹ۲쵽���Թ�����Һ����ɫ�������±仯����д����
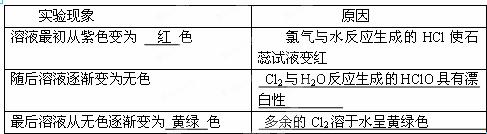
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ ɫ | ������ˮ��Ӧ���ɵ�HClʹʯ����Һ��� |
| �����Һ��Ϊ��ɫ | |
| �����Һ����ɫ��Ϊ ɫ | |


(2)
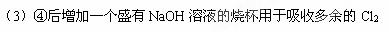
��

��ϰ��ϵ�д�
�����Ŀ


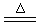 CuCl2 ���ڸ÷�Ӧ�У�
CuCl2 ���ڸ÷�Ӧ�У�