��Ŀ����
���ڱ�����A��B��C����ԭ���������������Ԫ�أ�A�ǵؿ��к������Ľ���Ԫ�أ�B�ļ۲�����Ų�Ϊnsn��1npn��1��C�������е�һ�ֳ��ý�������ԭ�ӵ���Χ�����Ų�Ϊ3d104s1��
(1)A��̬ԭ�ӵĵ����Ų�ʽΪ__________________��
(2)һ����̼(��N2��Ϊ�ȵ�����)�����ЦҼ���м���Ŀ֮��Ϊ________��
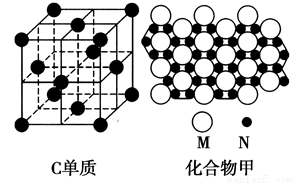
(3)C�ĵ��ʺ�ij��������۲��ֽṹ�ֱ���ͼ��ʾ��C����Χ�Ⱦ����Ҿ��������C����________�����Ļ�ѧʽΪ________(��M��N��ʾ)��
(4)����C�ڼ���ʱ��B������������Ӧˮ�����Ũ��Һ��Ӧ�Ļ�ѧ����ʽΪ____________��
(5)CԪ�ص�����������ˮ���������ڰ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
(6)����(N2H4)�Ǻ���ɴ����õĸ���ȼ�ϣ�����������Ϊԭ����ȡ����������KMnO4���������£�����[CO(NH2)2]�ʹ������ơ�NaOH��Һ��Ӧ����������Na2CO3��H2O������һ�ֲ��д���÷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
���״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϡ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶ȡ� | |
500 | 800 | ||
��2H2(g)+CO(g) | K1 | 2��5 | 0��15 |
��H2(g)+CO2(g) | K2 | 1��0 | 2��50 |
��3H2(g)+CO2(g) | K3 | ||
��1���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3=_______����K1��K2��ʾ����500��ʱ��÷�Ӧ����ijʱ�̣�H2(g)��CO2(g)��CH3OH(g)��H2O (g)��Ũ�ȣ�mol/L���ֱ�Ϊ0��8��0��1��0��3��0��15�� ���ʱ V��_____ V�棨� > ������=����<������
��2����3 L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c(CO)-��Ӧʱ��t�仯���ߢ���ͼ��ʾ������t0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ����ߢ��Ϊ���ߢ�ʱ���ı��������______________�������ߢ��Ϊ���ߢ�ʱ���ı��������_________________��

��3��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᡣͨ��״���£� ��a mol/L�Ĵ�����b mol/LBa(OH)2��Һ�������ϣ���Ӧƽ��ʱ��2c(Ba2��)= c(CH3COO��)���ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ________________��
������֪������һ�ֶ�Ԫ���ᣬ�������ƣ�NaHC2O4����Һ�����ԡ�
��1�������£���10 mL 0��01 mol��L-1 H2C2O4��Һ�еμ�10mL 0��01mol��L-1 NaOH��Һʱ���Ƚ���Һ�и�������Ũ�ȵĴ�С��ϵ_________________________ ��
��2����ȡ6��0g��H2C2O4��2H2O��KHC2O4��K2SO4����������ˮ�ܽ����250 mL ��Һ����ȡ���ݴ���Һ��25 mL���ֱ�����������ƿ�С���һ����Һ�м���2�η�̪��Һ���μ�0��25mol��L-1 NaOH ��Һ��20mLʱ����Һ����ɫ��Ϊdz��ɫ���ڶ�����Һ�μ�0��10 mol��L-1 ����KMnO4��Һ��16mLʱ��Ӧ��ȫ����ԭ������H2C2O4��2H2O�ĵ���������Ϊ_______ ��


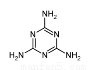
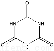 �����������������谷�����֮��ͨ��________��ϣ������������γɽ�ʯ��
�����������������谷�����֮��ͨ��________��ϣ������������γɽ�ʯ��
 -�ȱ����2-�ȱ��� D���ױ����ұ�
-�ȱ����2-�ȱ��� D���ױ����ұ�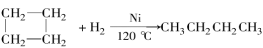
 ±���ⷢ�����Ʒ�Ӧ����̼ԭ��������4�Ļ�������±������ȡ����Ӧ������գ�
±���ⷢ�����Ʒ�Ӧ����̼ԭ��������4�Ļ�������±������ȡ����Ӧ������գ� 2SO3��g��, ���SO3Ũ���뷴Ӧ�¶ȹ�ϵ��ͼ������˵����ȷ����( )
2SO3��g��, ���SO3Ũ���뷴Ӧ�¶ȹ�ϵ��ͼ������˵����ȷ����( )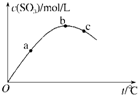
 CH3OH(g)
CH3OH(g)