��Ŀ����
DZ�ڴ�����ϡ��������� A �ǵڶ����������⻯���ͨ����λ���γɵĻ����������ĵȵ����壬��Է�������30.87��������Ϊ��ɫ���壬�ȶ��������ո��ڻ��� A �����ͷ�������ת��Ϊ������B����ϩ�ĵȵ����壩��B ���ȶ����ۺϳɾۺ��� C������ϩ�ĵȵ����壩��C ��155oC�ͷ�����ת��ΪX������Ȳ�ĵȵ����壩�����оۺ϶�Ϊ3�Ļ����� D �DZ��ĵȵ����塣����500oCʱ D �ͷ�������ת��Ϊ������ E��E �ж��־��ͣ������ƽ��ʯ��ԭ�Ӿ��壬Ҳ��������ʯī��Ƭ��ṹ�Ĺ����;��塣
��1�� д�� A��B��C��D��E �Ļ�ѧʽ��
��2��Ϊʹ A ��������������˻����� D ��ˮ��������������鷴Ӧ��д����ѧ����ʽ��
��1��A�� BNH6 B�� H2B��NH2���� BNH4��
C����[H2B��NH2]n�� D�� B3N3H6 E�� BN
��2�� 3CH4��2(HBNH)3��6H2O��3CO2��6H3BNH3
��������
�����������1��ԭ�����͵������ֱ���ȵ������ǵȵ����壬������D�DZ��ĵȵ����壬��D������������ѧʽ��B3N3H6������ΪA�������ǵȵ����壬��A��BNH6��B����ϩ�ĵȵ����壬��B��H2B��NH2���� BNH4����C�Ǿ���ϩ�ĵȵ����壬��C�ǣ�[H2B��NH2]n��������500oCʱ D �ͷ�������ת��Ϊ������ E������E��BN��
��2������ԭ���غ��֪�������ﻹ��CO2���ɣ����Է�Ӧ�Ļ�ѧ����ʽ��3CH4��2(HBNH)3��6H2O��3CO2��6H3BNH3��
���㣺����ȵ������Ӧ�á������ﻯѧʽ���ж��Լ���ѧ����ʽ����д��
�����������ѶȽϴ�ѧ������������������˸��ߵ�Ҫ���������Ŀ�����ǿ��ѧ����ȡ��Ϣ���ӹ���Ϣ���ӻ�ѧ�ӽ�ȥ�������ʵ���������������������������ѧ����ѧϰ��Ȥ������ѧ����ѧϰ�����ԣ��������ڼ���ѧ��̽����ѧ�������֪����

 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
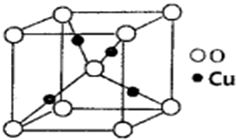 ij�����к��еĻ������ΪNi��Cu��Zn�Ļ���������ڶ�����̼������ȡ���ѣ��������Ҵ���ͬ���칹�壬���۵�-141.5�棬�е�-24.9�棬�ڼ��������¿ɷֽ�ɼ��顢���顢��ȩ�ȣ�
ij�����к��еĻ������ΪNi��Cu��Zn�Ļ���������ڶ�����̼������ȡ���ѣ��������Ҵ���ͬ���칹�壬���۵�-141.5�棬�е�-24.9�棬�ڼ��������¿ɷֽ�ɼ��顢���顢��ȩ�ȣ�