��Ŀ����
�����й�ʵ��ı����У�������ǣ� ��
| A����ȥ�Ҵ���ˮ����������ʯ�ң������ռ������ |
| B��������۵�ˮ������������������ˮ��Һ��ֱ�Ӽ�������Cu(OH)2��Һ��Ȼ����ȣ��۲��Ƿ��к�ɫ�������� |
| C����ȥ���������е�����ӱ���̼������Һ���������Һ����ˮ�� |
| D��������Һ�����ƣ��ڽྻ���Թ��м�2% AgNO3��Һ1��2 mL����μ���2%ϡ��ˮ���ߵα���������ǡ���ܽ�ʱΪֹ |
B
�������������A����ʯ�������Ƽ�����ˮ�����������ƣ���˳�ȥ�Ҵ���ˮ����������ʯ�ң������ռ�����T�ɣ�A��ȷ��B������ˮ�������������½��У���ȩ��������������ͭ����Һ�ķ�Ӧ���ڼ��������½��еģ���B����ȷ��C������̼������Һ���Խ��������������ܽ�ȣ��������ᣬ���Գ�ȥ���������е�����ӱ���̼������Һ���������Һ����ˮ�㣬C��ȷ��D���ڽྻ���Թ��м�2% AgNO3��Һ1��2 mL����μ���2%ϡ��ˮ���ߵα���������ǡ���ܽ�ʱΪֹ����ʱ������Һ��������Һ��D��ȷ����ѡB��
���㣺����ʵ�鷽�����������

��ʽ̼��ͭ��һ����;�㷺�Ļ���ԭ�ϡ���ҵ�Ͽ������Կ�ʴ��Һ����Ҫ����Cu2+��Fe2+��Fe3+��H +��Cl-���Ʊ������Ʊ��������£�
Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
| �� �� | Cu(OH)2 | Fe (OH)2 | Fe (OH)3 |
| ��ʼ����pH | 4.2 | 5.8 | 1.2 |
| ��ȫ����pH | 6.7 | 8.3 | 3.2 |
��1����������Ҫ�ɷ��� ��д��ѧʽ����
��2�����ڷ�ӦA����Һ��pH��ΧӦΪ ��ѡ����Լ�����ʵ��� ������ţ���
a����ˮ b��ϡ���� c���������� d��̼��ͭ
��3����ӦB���¶�����ߣ�����������ɫ��Ʒ�п��ܻ���ֵ������� ��
��4����Na2CO3��Һ���뵽һ����CuCl2��Һ�еõ�����������Ӧ�����ӷ���ʽ��ʾ��
�� ����ֻ��CuCO3�� ��
�� ����ֻ��Cu(OH)2�ҷų����ݣ� ��
��5����ʽ̼��ͭ����ɿɱ�ʾΪ��aCuCO3?bCu(OH)2?cH2O��Ҫ�ⶨ����ɣ���ͬѧ��Ƶ�ʵ�鷽������Ҫ����������裺�ٳ�����Ʒ���������ڸ��·ֽ⣻�۲��CO2���������ܲ��ˮ�������������ݳ���CuO������������ͬѧ��Ϊ��������⣬ʵ��ֻ��ⶨ�ĸ����е��������ɣ�����������Ϊ ������ţ�дһ�鼴�ɣ���
��������װ�úͲ������ܴﵽʵ��Ŀ�ĵ���
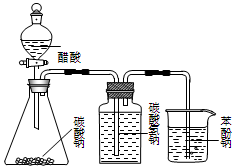


�� �� �� ��
| A����װ�ã�����Ũ������Ҵ��������Ϊ��3��1�����Һ����ȡ��ϩ |
| B����װ�ã��Ƚϴ��ᡢ̼�ᡢ�������ߵ�����ǿ�� |
| C����װ�ã�����б��ӵı��м���NaOH��Һ����ȥ���еı��� |
| D����װ�ã�A��Ϊ�Ҵ������ᣬBΪ����̼������Һ����ȡ�������� |
����ʵ��������ܴﵽ���ӦĿ�ĵ���
| ��� | ʵ������ | ʵ��Ŀ�� |
| A�� | ��ʢ��10��0��1 mol/L AgNO3��Һ���Թ��еμ�0��1 mol/L NaCl��Һ���������г������ɣ��������еμ�0��1 mol/L Na2S��Һ | ֤��AgCl������ת��Ϊ�ܽ�ȸ�С��Ag2S���� |
| B�� | ��2 mL�ױ��м���3��KMnO4������Һ������2mL�����м���3��KMnO4������Һ���� | ֤���뱽�������ļ��ױ����� |
| C�� | ��Na2SiO3��Һ��ͨ��CO2 | ֤��̼������Աȹ���ǿ |
| D�� | �ڵ�����Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ������ | ��֤������ˮ�� |
�����й�ʵ�������������ʹ����ȷ���� 
A B C D
| A����ȥ�����е��Ȼ��� | B���Ʊ�Fe(OH)2 |
| C��ʵ�����Ʊ����� | D������Ũ���� |
����ʵ�鲻�ܴﵽԤ��ʵ��Ŀ�ĵ��ǣ� ��
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��ʢ��10��0.1 mol��L��1 AgNO3��Һ���Թ��еμ�0.1 mol��L��1 NaCl��Һ���������г������ɣ��������еμ�0.1 mol��L��1 Na2S��Һ | ֤��AgCl��ת��Ϊ�ܽ�ȸ�С��Ag2S |
| B | ��2 mL�ױ��м���3������KMnO4��Һ������2 mL���м���3������KMnO4��Һ���� | ֤���뱽�������ļ��ױ� ���� |
| C | ��Na2SiO3��Һ��ͨ��CO2 | ֤��̼������Աȹ���ǿ |
| D | �������Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ����Һ������ | ��֤������ˮ�� |
����ͼװ�òⶨˮ���⡢��Ԫ�ص������ȣ��䷽���Ƿֱ�ⶨͨ����ǰ�����ܵ��������U�ιܵ������ʵ����m(H)��m(O)>1��8�����жԵ�����һ�����ԭ��ķ����У�һ���������(����)
| A����װ��֮��ȱ�ٸ���װ�� |
| B����װ�ú�ȱ�ٸ���װ�� |
| C����װ���в���������ˮ���� |
| D��CuOû��ȫ������ԭ |
����װ�ò�����ɵ�ʵ����(����)
| | A | B | C | D |
| װ �� |  |  |  |  |
| ʵ �� | ���������Բ���п������ķ�Ӧ���� | �Ʊ����ռ�����NO���� | ��֤�¶ȶԻ�ѧƽ���Ӱ�� | �������ⸯʴʵ�� |
