��Ŀ����
�йص������Һ������������˵������ȷ����
A��0.2 mol��L��1��ˮ�У�c(OH��)��c(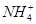 )��� )��� |
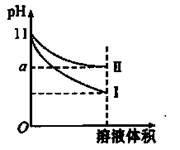
| B��10 mL 0.02 mol��L��1 HCl��Һ��10 mL 0.02 mol��L��1 Ba(OH)2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20 mL������Һ��pH��12 |
| C��0.1 mol/L��Na2S��Һ�У�c(OH��)��c(H��)��c(HS��)��c(H2S) |
| D��pH��3��һԪ���pH��11��һԪ��������ͺ����Һ��һ����c(OH��)��c(H��) |
B
��ˮ����Ҫ�ɷ���NH3��H2O��NH3��H2O��һԪ����������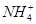 ��OH����H2OҲ�����OH������c(NH) <c(OH��)��Aѡ�����
��OH����H2OҲ�����OH������c(NH) <c(OH��)��Aѡ�����
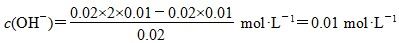 ����pH����12��Bѡ����ȷ���������غ�֪��c(OH��)��c(H��)��c(HS��)��2c(H2S)��ѡ��C����D�����ǿ����ǿ������c(OH��)��c(H��)������ǿ���������c(H��)<c(OH��)������������ǿ���c(H��)��c(OH��)������
����pH����12��Bѡ����ȷ���������غ�֪��c(OH��)��c(H��)��c(HS��)��2c(H2S)��ѡ��C����D�����ǿ����ǿ������c(OH��)��c(H��)������ǿ���������c(H��)<c(OH��)������������ǿ���c(H��)��c(OH��)������
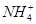 ��OH����H2OҲ�����OH������c(NH) <c(OH��)��Aѡ�����
��OH����H2OҲ�����OH������c(NH) <c(OH��)��Aѡ�����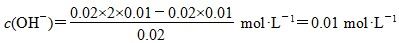 ����pH����12��Bѡ����ȷ���������غ�֪��c(OH��)��c(H��)��c(HS��)��2c(H2S)��ѡ��C����D�����ǿ����ǿ������c(OH��)��c(H��)������ǿ���������c(H��)<c(OH��)������������ǿ���c(H��)��c(OH��)������
����pH����12��Bѡ����ȷ���������غ�֪��c(OH��)��c(H��)��c(HS��)��2c(H2S)��ѡ��C����D�����ǿ����ǿ������c(OH��)��c(H��)������ǿ���������c(H��)<c(OH��)������������ǿ���c(H��)��c(OH��)������
��ϰ��ϵ�д�
�����Ŀ
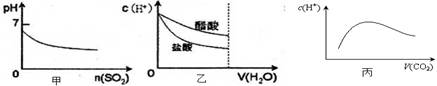
 ?[B(OH)4]��(aq)��H��(aq)������˵����ȷ����
?[B(OH)4]��(aq)��H��(aq)������˵����ȷ����