��Ŀ����
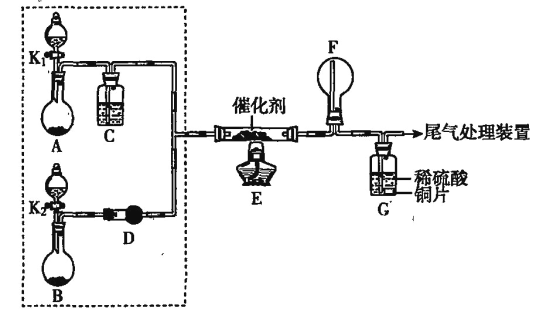
����Ŀ��ijС��ͬѧ��̽��NH3�Ĵ�������Ӧ������ͼװ�ý���ʵ�顣A��Bװ�ÿ�ѡ�õ�ҩƷ:Ũ��ˮ��H2O2��Һ������ˮ��NaOH���塢MnO2��
��1��NH3�������Ļ�ѧ����ʽ��__________��
��2���ס�����ͬѧ�ֱ���ͼװ�ý���ʵ�顣һ��ʱ�����װ��G�е���Һ�������ɫ��
�ټ۲쵽װ��F���к���ɫ���������ɺ���ɫ����Ļ�ѧ����ʽ��__________��
���ҹ۲쵽װ��F��ֻ�а������������̵ijɷ���__________(�ѧʽ)��
�������ӷ���ʽ����װ��G����Һ�����ɫ��ԭ��:__________��
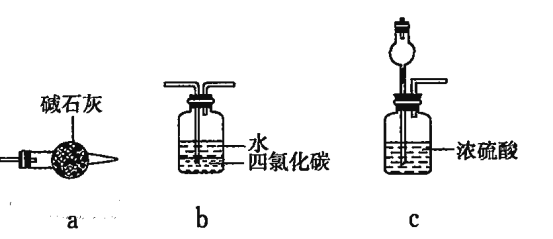
��3��Ϊ������ʵ����װ��F��Ҳ�۲쵽����ɫ����������ԭʵ��Ļ����Ͻ��иĽ�:
�ټ���Ϊ�ɵ���K1��K2����A��Bװ���еIJ�������Ӧ__________(��������������������)װ��A�еIJ���������__________(��������������������)װ��B�еIJ�������
������Ϊ����װ��E��F������������װ�ÿ�����__________(����ĸ���)��
��4��Ϊʵ�ָ÷�Ӧ��Ҳ������ͼ��ʾ��װ���滻��.��װ�������߿���
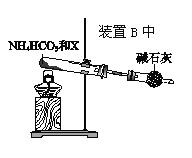
������XΪ__________��NH4HCO3��������__________��
���𰸡�4NH3+5O2![]() 4NO+6H2O2NO + O2
4NO+6H2O2NO + O2![]() 2NO2NH4NO33Cu + 8H+ +2NO3- =3Cu2++2NO�� +4H2O���Ӽ���abcNa2O2NH4HCO3���ȷֽ�ų�NH3��ͬʱ�ų���CO2 ��H2O�ֱ���Na2O2��Ӧ����O2
2NO2NH4NO33Cu + 8H+ +2NO3- =3Cu2++2NO�� +4H2O���Ӽ���abcNa2O2NH4HCO3���ȷֽ�ų�NH3��ͬʱ�ų���CO2 ��H2O�ֱ���Na2O2��Ӧ����O2
��������
��1�����Ĵ�������Ӧ��������-3�۵ĵ���������������+2�۵�һ����������ѧ��Ӧ����ʽΪ��4NH3+5O2![]() 4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��
��2���ٰ��������˴������IJ���һ��������һ������������������Ϊ������������2NO+O2=2NO2������װ��F���к���ɫ����������ʴ�Ϊ��2NO+O2�T2NO2��
���ҹ۲쵽װ��F��ֻ�а������ɣ����������ڰ��������ᷴӦNH3+HNO3=NH4NO3�������˰�ɫ��NH4NO3���壬�ʴ�Ϊ��NH4NO3��
����װ��G�У����ɵĶ���������ˮ��Ӧ���������һ����������ѧ����ʽΪ��3NO2+H2O�T2HNO3+NO��������ᣬ����ͭ�����ᷴӦ��3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2Oʵ���ǣ�3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��3����Ϊ�˳�������������������������������������������ٰ���������������ĸ���װ�ÿ�֪��AΪ��������װ�á�BΪ��ȡ������װ�ã��ʿ�����װ��A�еIJ����������װ��B�еIJ��������ʴ�Ϊ�����ӣ� ���٣�
��Eװ���ǰ����Ĵ�����װ�ã���װ���г������������������Ͱ�����һ��������ˮ��������4NO+2H2O+3O2=4HNO3��NH3+HNO3=NH4NO3�����Ա���F����������泥�ʵ����F�п�������ɫ�Ķ������������ȥˮ����������װ��E��F������һ��װ�ã���װ�ÿ��Խ�������ˮ��ȥ��a��ʯ������ˮ����������ͨ����������bװ�����հ���������ͨ��Ũ�������հ�����ˮ������ʵ����F�п�������ɫ�Ķ�����������ѡabc���ʴ�Ϊ��abc��
��4��Ϊʵ��2NO+O2�T2NO2�÷�Ӧ��������������̼��������ȷֽ����ɰ�����ˮ�Ͷ�����̼���ų���CO2��H2O��Na2O2��Ӧ����O2����XΪNa2O2���ʴ�Ϊ��Na2O2��NH4HCO3���ȷֽ�ų�NH3��ͬʱ�ų���CO2 ��H2O�ֱ���Na2O2��Ӧ����O2��

 ��У����ϵ�д�
��У����ϵ�д�