��Ŀ����
�ݱ���������ԱӦ�ü����ģ����ṹ����C60������N60����֪��
��N60������ÿ����ԭ�Ӿ���N-N���������Nԭ�Ӷ��γ�8�����ȶ��ṹ��
��N-N���ļ���Ϊ167kJ?mol-1��
��ش��������⣮
��1��N60������ɵľ���Ϊ ���壬���ۡ��е��N2 ����ߡ��͡�����N60����Ϊ ���ӣ���Ի�Ǽ��ԣ���
��2��N60������ɵľ����д��ڵ��������� ��
A�����ۼ� B�����Ӽ� C����� D�����»��� E����λ��
��3��C60�е�̼Ԫ����N60�еĵ�Ԫ�ؿ��γ�һ��CN?���ӣ���������N2��Ϊ�ȵ����壬д�������ӵĵ���ʽ ��
��4����֪N��N���ļ���Ϊ942kJ?mol-1����1mol N60�ֽ��N2ʱ���ջ�ų��������� ��
��N60������ÿ����ԭ�Ӿ���N-N���������Nԭ�Ӷ��γ�8�����ȶ��ṹ��
��N-N���ļ���Ϊ167kJ?mol-1��
��ش��������⣮
��1��N60������ɵľ���Ϊ
��2��N60������ɵľ����д��ڵ���������
A�����ۼ� B�����Ӽ� C����� D�����»��� E����λ��
��3��C60�е�̼Ԫ����N60�еĵ�Ԫ�ؿ��γ�һ��CN?���ӣ���������N2��Ϊ�ȵ����壬д�������ӵĵ���ʽ
��4����֪N��N���ļ���Ϊ942kJ?mol-1����1mol N60�ֽ��N2ʱ���ջ�ų���������
���㣺��ͬ����Ľṹ��������������������,��Ӧ�Ⱥ��ʱ�
ר�⣺��ѧ��Ӧ�е������仯,��ѧ���뾧��ṹ
��������1�����ݾ���Ĺ������жϣ����Ӿ������Է�������Խ��е�Խ�ߣ��ǽ��������γɷǼ��Է��ӣ�
��2�����ݾ��������ж���������
��3�����ݵ������ӵĵ���ʽд��CN-�ĵ���ʽ��ע����������ӣ�
��4���ȼ���N60��N-N������Ŀ�ټ����ܼ��ܣ�
��2�����ݾ��������ж���������
��3�����ݵ������ӵĵ���ʽд��CN-�ĵ���ʽ��ע����������ӣ�
��4���ȼ���N60��N-N������Ŀ�ټ����ܼ��ܣ�
���
�⣺��1��N60����Ĺ�����Ϊ���ӣ��������ڷ��Ӿ��壻���Ӿ������Է�������Խ���ۡ��е�Խ�ߣ�����N60���ۡ��е��N2�ߣ�N60���ɷǽ��������γɵķǼ��Է��ӣ�
�ʴ�Ϊ�����ӣ��ߣ��Ǽ��ԣ�
��2��N60����Ĺ�����Ϊ���ӣ����ڷ��Ӿ��壬���Ӿ����д��ڷ��»�����N��N֮����ڹ��ۼ����ʴ�Ϊ��AD��
��3�����ݵ������ӵĵ���ʽд��CN-�ĵ���ʽ����CN-�����ӣ��������ӵ���ʽ����д�������������ʽΪ��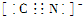 ���ʴ�Ϊ��
���ʴ�Ϊ��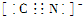 ����4��ÿ��Nԭ�Ӿ��Ե����������������ԭ�ӣ�ÿ����������2����ԭ�ӹ��ã�ÿ����ԭ����1.5����������1��N60���ӵĽṹ�к���90��N-N������1molN60���ܼ���Ϊ167kJ?mol-1��90mol=15030KJ������30molN��N���ļ���Ϊ942kJ?mol-1��30mol=28260KJ����Ӧ�ų�����Ϊ28260KJ-15030KJ=13230 KJ��
����4��ÿ��Nԭ�Ӿ��Ե����������������ԭ�ӣ�ÿ����������2����ԭ�ӹ��ã�ÿ����ԭ����1.5����������1��N60���ӵĽṹ�к���90��N-N������1molN60���ܼ���Ϊ167kJ?mol-1��90mol=15030KJ������30molN��N���ļ���Ϊ942kJ?mol-1��30mol=28260KJ����Ӧ�ų�����Ϊ28260KJ-15030KJ=13230 KJ��
�ʴ𰸣��ų�13230 KJ��
�ʴ�Ϊ�����ӣ��ߣ��Ǽ��ԣ�
��2��N60����Ĺ�����Ϊ���ӣ����ڷ��Ӿ��壬���Ӿ����д��ڷ��»�����N��N֮����ڹ��ۼ����ʴ�Ϊ��AD��
��3�����ݵ������ӵĵ���ʽд��CN-�ĵ���ʽ����CN-�����ӣ��������ӵ���ʽ����д�������������ʽΪ��
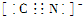 ���ʴ�Ϊ��
���ʴ�Ϊ��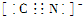 ����4��ÿ��Nԭ�Ӿ��Ե����������������ԭ�ӣ�ÿ����������2����ԭ�ӹ��ã�ÿ����ԭ����1.5����������1��N60���ӵĽṹ�к���90��N-N������1molN60���ܼ���Ϊ167kJ?mol-1��90mol=15030KJ������30molN��N���ļ���Ϊ942kJ?mol-1��30mol=28260KJ����Ӧ�ų�����Ϊ28260KJ-15030KJ=13230 KJ��
����4��ÿ��Nԭ�Ӿ��Ե����������������ԭ�ӣ�ÿ����������2����ԭ�ӹ��ã�ÿ����ԭ����1.5����������1��N60���ӵĽṹ�к���90��N-N������1molN60���ܼ���Ϊ167kJ?mol-1��90mol=15030KJ������30molN��N���ļ���Ϊ942kJ?mol-1��30mol=28260KJ����Ӧ�ų�����Ϊ28260KJ-15030KJ=13230 KJ���ʴ𰸣��ų�13230 KJ��
���������⿼���˾������͵��жϡ��е�ıȽϡ���ѧ��������ʽ������������ȣ���Ŀ�Ѷ��еȣ���Ҫѧ���Գ������ʵĽṹ�������������գ�

��ϰ��ϵ�д�
 ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ
ͬ��ͬѹ�£��ڵ������Ķ�����������Ͷ�����̼����ıȽ��У���ȷ���ǣ�������
| A���ܶȱ�Ϊ11��16 |
| B�����ʵ�����Ϊ16��11 |
| C�������Ϊ11��16 |
| D�����Ӹ�����Ϊ16��11 |
���CuCl2��NaCl�Ļ����Һ�����������������ȷֱ������������ǣ�������
| A��H2��Cl2 |
| B��Cu��Cl2 |
| C��H2��O2 |
| D��Cu��O2 |
�л���ķ����У������������е�һ�ֹ�����ʱ�����ܷ���ȡ����Ӧ��������Ӧ��������Ӧ�����ܷ�����ȥ��Ӧ���ǣ�������
| A��-COOH |
B�� |
| C��-OH |
D��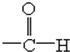 |
���з�Ӧ�����������ɵ��ǣ�������
| A��Na2SiO3��Һ�м���������� |
| B��NaAlO2��Һ��ͨ�����CO2 |
| C������Na2CO3��Һ��ͨ�������CO2 |
| D��AgNO3��Һ�еμӰ�ˮ������ |
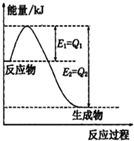 ��1�����ᣨH3BO3����Һ�д�������ƽ�⣺
��1�����ᣨH3BO3����Һ�д�������ƽ�⣺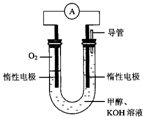 ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�ã�
ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�ã�