��Ŀ����
����Ŀ�����ڷ��֣�H2S�Ǽ�NO��CO֮��ĵ�����������ϵ�����źŷ��ӣ������в���������źŴ��ݡ�����Ѫ�ܼ����Ѫѹ�Ĺ��ܡ��ش��������⣺
��1��������ʵ�У����ܱȽ��������������������ǿ������_____�����ţ���
A�����������̼��������Һ��Ӧ�������������
B������������ȶ���ǿ��������
C��ͬŨ�ȵ���������������pHΪǰ�ߴ��ں���
D��������Ļ�ԭ��ǿ��������
��2����ͼ��ͨ���Ȼ�ѧѭ���ڽϵ��¶�����ˮ������ֽ��Ʊ������ķ�Ӧϵͳԭ����

ͨ�����㣬��֪ϵͳ����ϵͳ����������Ȼ�ѧ����ʽ�ֱ�Ϊ________________��___________���Ƶõ���H2�����������ٵ���_______��
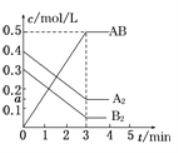
��3��һ����������ˮ��Һ��1 mol Cl����ClO x��(x��1��2��3��4)������(KJ)��Դ�С��ͼ��ʾ��

��D��______________________ (�����ӷ���)��
��B��A��C��Ӧ���Ȼ�ѧ����ʽΪ______________________ (�����ӷ��ű�ʾ)��
���𰸡� BD H2O(l)=H2(g)+![]() O2(g) ��H=+286 kJ/mol H2S(g)=H2(g)+S(s) ��H=+20 kJ/mol ϵͳ��II�� ClO4�� 3 ClO��(
O2(g) ��H=+286 kJ/mol H2S(g)=H2(g)+S(s) ��H=+20 kJ/mol ϵͳ��II�� ClO4�� 3 ClO��(![]() )==ClO3��(
)==ClO3��(![]() )+2Cl��(
)+2Cl��(![]() ) ��H=
) ��H=![]() 117KJ��mol��1
117KJ��mol��1
����������1��A�����ݸ��ֽⷴӦ�Ĺ��ɣ�ǿ��+������=ǿ����+���ᣬ��֪����H2SO3>H2CO3>H2S��ѡ��A����B�������������������ȶ��Բ������ˮ���ɶ�Ӧ�����������Ƚ������ᡢ����������ǿ����ѡ��B����C����Ũ�ȵĶ�Ԫ���ᣬ����������Խ����Һ������Խǿ������PH��ԽС��������������Һ��PH�ȵ�Ũ�ȵ��������С������֤�����ԣ�H2SO3> H2S��ѡ��C����D�����ʵĻ�ԭ�Դ�С������Ԫ�صĻ��ϼۼ����ṹ�йأ����������������ӵ�Ũ�ȴ�С�أ���˲���֤�����ߵ�����ǿ����ѡ��D��ȷ����ѡBD��
��2����H2SO4(aq)= SO2(g)+ H2O(l) S(s)+![]() O2(g) ��H1=��327 kJ/mol
O2(g) ��H1=��327 kJ/mol
��SO2(g)+I2(s)+ 2H2O(l)=2HI(aq)+ H2SO4(aq) ��H2=��151 kJ/mol
��2HI(aq)= H2 (g)+ I2(s) ��H3=��110 kJ/mol
��H2S(g)+ H2SO4(aq)=S(s)+SO2(g)+ 2H2O(l) ��H4=��61 kJ/mol
��+��+�ۣ������ɵ�ϵͳ��I�����Ȼ�ѧ����ʽH2O(l)=H2(g)+![]() O2(g) ��H=+286 kJ/mol��
O2(g) ��H=+286 kJ/mol��
��+��+�ܣ������ɵ�ϵͳ��II�����Ȼ�ѧ����ʽH2S (g)=H2(g)+S(s) ��H=+20 kJ/mol��
����ϵͳ����ϵͳ�����Ȼ�ѧ����ʽ��֪��ÿ��Ӧ����1mol�������������յ�������ǰ���٣�������ȡ���������������������ٵ���ϵͳ��II��
��3���ٸ���ͼʾ���߿�֪ClԪ�صĻ��ϼ�Խ��Խ�ߣ������߿�֪D��ClԪ�صĻ��ϼ��ǣ�7�ۣ����ClOx����x��4����DΪClO4�����ڸ������߿�֪AΪCl����BΪClO�����ʻ�ѧ����ʽΪ3ClO��=2Cl����ClO3-��������߿�֪ClO�����Cl��������Ϊ60 kJ��ClO3�����Cl��������Ϊ63 kJ����˸÷�Ӧ�ķ�Ӧ��Ϊ��H��(63��60) kJ��mol��1��2��60 kJ��mol��1����117 kJ��mol��1��