��Ŀ����
ʵ�飺��������������(FeSO4?7H2O)������ͼ1����ʾ�ĸ����Թ�A�����������ǿ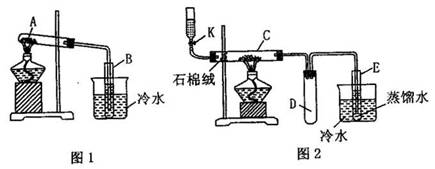
��ַ�Ӧ���Թ�A�еò�������ף����Թ�B�ڵõ���ɫҺ���ҡ�ȡ������ͼ2�е�ʯӢ��C�У����Ӻ��������ֵ�������������K����εμ��Ҵ�ʹC��ʯ����(һ����ά״�����β��ϣ����ȶ�����ȼ��)�������㣬�ر�K��Ȼ����ȣ�����������(���ȹ����л����ԽϿ�ؿ������ر�����K�������Ҵ���ʹ֮ͨ��ʯ������)���ɹ۲쵽����ɫ�ɺ���ɫ��Ϊ��ɫ����Ӧֹͣ��ȡ�Թ�E�е�Һ��0.5ml����μ��뵽�������Ƶ�������Һ���Թ�F�У����Թܷ���ˮԡ�����ȣ����û�ۿ����Թ��ڱ��ϸ���һ������羵�Ľ�������
�ش��������⣺
(1)д����ͼ1��ʾ�ĸ����Թ�A�з�����Ӧ�Ļ�ѧ����ʽ��_____________________________��
(2)���ݷ�Ӧԭ����������ͼ1ʵ��װ�õ���Ҫȱ��_____________________________________��
(3)�Թ�C�з�Ӧ�Ļ�ѧ����ʽΪ��________________________________________��
(4)������μ���ͼ2��ʾװ�������ԣ�__________________________________��
(5)�Թ�D��װ�ڴ˵������ǣ�_______________________________________��
(6)д��E�Թ��з�Ӧ�Ļ�ѧ����ʽ��__________________________________��
 ��У����ϵ�д�
��У����ϵ�д�




