��Ŀ����
������ij�о�С��̽��Ӱ�컯ѧ��Ӧ������һЩ���ص�������ݣ�����ͨ��ָ20�棻��| ʵ����� | ����������ҺŨ��/% | ����������Һ����/g | �¶�/�� | ������������/g | �������/ml | ��Ӧ����ʱ��/s |
| �� | 5 | 12 | 20 | 0.2 | 125 | 11 |
| �� | 30 | 12 | 20 | 0.2 | 125 | 2 |
| �� | 30 | 12 | 40 | / | 125 | 148 |
| �� | 30 | 12 | 90 | / | 125 | 82 |
��2��ͨ���Ա�ʵ��
��3��������ʵ���֪��ʵ�����ù���������ȡ����������ʵ�������
��2���Ƚϱ����еĢۢ�������������������ͬʱ�����Կ����¶ȶԷ�Ӧ���ʵ�Ӱ�죻
��3������ʵ���Ҳ����������ȡ�������ռ���ԭ�����ѡ�ɣ�
�ʴ�Ϊ����������Ũ�ȣ�
��2���ۢ������¶Ȳ�ͬ����������ͬ�������Ƕ��¶�Ӱ�컯ѧ��Ӧ�ٶȵ�̽�����ӽ�����¶ȸ�ʱ��Ӧ���õ�ʱ��϶̣��ȷ�Ӧ���ٶȽϿ죬�¶ȵ�ʱ��Ӧ���ٶ�����
�ʴ�Ϊ���ۢܣ��¶�Խ�߷�Ӧ�ٶ�Խ�죬�¶�Խ�ͷ�Ӧ�ٶ�Խ����
��3��ʵ�����ù���������ȡ����Ҫ��Ӧ����ײ��������Բ��ò����ȶ��Ӵ����ķ������нϺ��ʣ��������ռ��ĽǶ�֪����Ӧ���ٶȲ���̫��Ҳ����̫������������ʵ�������20��ʱ5%����������Һ�м��������������̣�
�ʴ�Ϊ��20��ʱ5%����������Һ�м��������������̣�


��1��MgCO3�ķֽ����Ϊ
��2��װ��C��������
��3�����о�С���Ϊ���飬������ͼװ�ý��жԱ�ʵ�飬�����þƾ��ơ������þƾ���ƶ�װ��D���ȣ���Ӧ�����Ϊ��ɫ��ĩ�������������ֱ��ò����������ʵ�飺
| ���� | ���� | �������� | �������� |
| 1 | ȡ��ɫ��ĩ�������� | �ܽ⣬������ | �ܽ⣬������ |
| 2 | ȡ����1����Һ���μ�K3[Fe��CN��6]��Һ | ��ɫ���� | ��ɫ���� |
| 3 | ȡ����1����Һ���μ�KSCN��Һ | ��� | ������ |
| 4 | ȡ����3��Һ�еμ�������ˮ | ��ɫ��ȥ | �ȱ�죬����ɫ |
�ڼ��鲽��1�з�Ӧ�����ӷ���ʽΪ
�����鲽��4�У���Һ����ԭ��Ϊ
�ܴ�ʵ�鰲ȫ���ǣ�����ͼװ�ÿɲ�ȡ�ĸĽ���ʩ��
��11�֣�������ijѧϰС����Ҷ����ijЩ���ʽ����о���ѧϰ�Ĺ��̣�
[�о�����]̽���Ҷ����ijЩ����
[��������]�Ҷ��ᣨHOOC��COOH���׳Ʋ��ᣬ����Ҫ�����������£�
| ���� | �Ҷ��� | �Ҷ��ᾧ�� |
| ����ʽ | H2C2O4 | H2C2O4��2H2O |
| ��ɫ״̬ | ��ɫ���� | ��ɫ���� |
| �ܽ�ȣ�g�� | 8.6��20�棩 | �� |
| �۵㣨�棩 | 189.5 | 101.5 |
| �ܶȣ�g��cm��3�� | 1.900 | 1.650 |
������100��ʱ��ʼ������157��ʱ��������������ʼ�ֽ⡣
����Ʋ�����ˮ��
����������ʹ����ʯ��ˮ����ǡ�
���������ڵ����¿�����Ϊ���塣
�������������ṩ����Ϣ���ش��������⣺
[�������]
������һ�����ݲ��ᾧ�����ɶ���ֽ������в���
��Ʒ�����
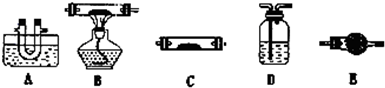
��1����С��ͬѧ���������ΪCO��CO2��H2O����������װ�����һ��̽��ʵ��װ�ã����ᾧ��ֽ�װ���ԣ�װ�ÿ��ظ�ʹ�ã����ӵ�����ȥ����

A��ˮ��װ��ˮ����B��װ����ͭ��C��װ��ˮ����ͭ��D��װ����ʯ��ˮ��E��װ��ʯ��
��ش��������⣺
��װ�õ�����˳��Ϊ��A��_____________________________________________��
�ڼ��������CO��ʵ��������____________________________________________________________
������װ���Ƿ���ڲ�����֮���� �����ǻ�����и���ν��___________________________________________________________________________
����������Ҷ������������
��Ʒ�����
��2����С��ͬѧΪ��֤����������������������ʵ�飬�����ܴﵽʵ��Ŀ����______������ĸ����
A�������ᾧ�����ں���̪��NaOH��Һ�У���Һ��ɫ
B���ⶨ��ͬŨ�ȵIJ����������Һ��pH
C���ⶨ�����ƣ�Na2C2O4����Һ��pH
D����������Һ����Na2CO3��Һ�У���CO2�ų�
�����������Ҷ�����л�ԭ��
��Ʒ�����
��3����С��ͬѧ���������ữ��KMnO4��Һ�е�������IJ�����Һ����������KMnO4��Һ��ɫ���Ӷ��жϲ�����н�ǿ�Ļ�ԭ�ԡ���ƽ�÷�Ӧ�����ӷ���ʽ��
___MnO4��+___H2C2O4 +___H+ ===___Mn2+ +___CO2��+___H2O
��4����������ԭ���ɶ����ⶨij���ᾧ����Ʒ������H2C2O4��2H2O������һЩ���ʣ���H2C2O4��2H2O�ĺ�����
�����ǣ���ȡ����Ʒ0.12 g��������ˮ��ȫ�ܽ⣬Ȼ����0.020 mol��L��1
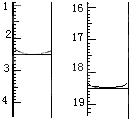
������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ�����ζ�ǰ��ζ����е�Һ�������ͼ��ʾ����λ��mL������ò��ᾧ����Ʒ��H2C2O4��2H2O����������Ϊ_____________��
����֪���ԭ��������Mr(H2C2O4��2H2O)=126��

��11�֣�������ijѧϰС����Ҷ����ijЩ���ʽ����о���ѧϰ�Ĺ��̣�
[�о�����]̽���Ҷ����ijЩ����
[��������]�Ҷ��ᣨHOOC��COOH���׳Ʋ��ᣬ����Ҫ�����������£�
|
���� |
�Ҷ��� |
�Ҷ��ᾧ�� |
|
����ʽ |
H2C2O4 |
H2C2O4��2H2O |
|
��ɫ״̬ |
��ɫ���� |
��ɫ���� |
|
�ܽ�ȣ�g�� |
8.6��20�棩 |
�� |
|
�۵㣨�棩 |
189.5 |
101.5 |
|
�ܶȣ�g��cm��3�� |
1.900 |
1.650 |
��֪��
������100��ʱ��ʼ������157��ʱ��������������ʼ�ֽ⡣
����Ʋ�����ˮ��
����������ʹ����ʯ��ˮ����ǡ�
���������ڵ����¿�����Ϊ���塣
�������������ṩ����Ϣ���ش��������⣺
[�������]
������һ�����ݲ��ᾧ�����ɶ���ֽ������в���
��Ʒ�����
��1����С��ͬѧ���������ΪCO��CO2��H2O����������װ�����һ��̽��ʵ��װ�ã����ᾧ��ֽ�װ���ԣ�װ�ÿ��ظ�ʹ�ã����ӵ�����ȥ����

A��ˮ��װ��ˮ����B��װ����ͭ��C��װ��ˮ����ͭ��D��װ����ʯ��ˮ��E��װ��ʯ��
��ش��������⣺
�� װ�õ�����˳��Ϊ��A��_____________________________________________��
�� ���������CO��ʵ��������____________________________________________________________
�� ����װ���Ƿ���ڲ�����֮���� �����ǻ�����и���ν��___________________________________________________________________________
����������Ҷ������������
��Ʒ�����
��2����С��ͬѧΪ��֤����������������������ʵ�飬�����ܴﵽʵ��Ŀ����______������ĸ����
A�������ᾧ�����ں���̪��NaOH��Һ�У���Һ��ɫ
B���ⶨ��ͬŨ�ȵIJ����������Һ��pH
C���ⶨ�����ƣ�Na2C2O4����Һ��pH
D����������Һ����Na2CO3��Һ�У���CO2�ų�
�����������Ҷ�����л�ԭ��
��Ʒ�����
��3����С��ͬѧ���������ữ��KMnO4��Һ�е�������IJ�����Һ����������KMnO4��Һ��ɫ���Ӷ��жϲ�����н�ǿ�Ļ�ԭ�ԡ���ƽ�÷�Ӧ�����ӷ���ʽ��
___MnO4��+___H2C2O4 +___H+ ===___Mn2+ +___CO2��+___H2O
��4����������ԭ���ɶ����ⶨij���ᾧ����Ʒ������H2C2O4��2H2O������һЩ���ʣ���H2C2O4��2H2O�ĺ�����
�����ǣ���ȡ����Ʒ0.12 g��������ˮ��ȫ�ܽ⣬Ȼ����0.020 mol��L��1
������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ�����ζ�ǰ��ζ����е�Һ�������ͼ��ʾ����λ��mL������ò��ᾧ����Ʒ��H2C2O4��2H2O����������Ϊ_____________��
����֪���ԭ��������Mr(H2C2O4��2H2O) =126��
=126��