ƒøƒ⁄»ð
£®10∑÷£©”…º∏÷÷¿Î◊”ªØ∫œŒÔ◊È≥…µƒªÏ∫œŒÔ£¨∫¨”–“‘œ¬¿Î◊”÷–µƒ»Ù∏…÷÷£∫K+°¢NH4+°¢Mg2+°¢Cu2+°¢Ba2+°¢C1°™°¢SO42°™°¢CO32-°£Ω´∏√ªÏ∫œŒÔ»Ð”⁄ÀÆ∫Ûµ√ŒÞ…´≥Œ«Â»Ð“∫£¨œ÷∑÷±»°3∑ð100mL∏√»Ð“∫Ω¯––»Áœ¬ µ—È£∫
‘ªÿ¥œ¬¡–Œ £∫
£®1£©∏√ªÏ∫œŒÔ÷–“ª∂®≤ª¥Ê‘⁄µƒ¿Î◊” « °£
£®2£©»Ð“∫÷–“ª∂®¥Ê‘⁄µƒ“ı¿Î◊” « °£
£®3£© ‘–¥≥ˆ µ—Èb∑¢…˙∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω °£
£®4£©≈–∂œªÏ∫œŒÔ÷– «∑ҥʑ⁄K+£ø £®ÃÓ°∞ «°±ªÚ°∞∑Ò°±£©°£
¿Ì”… « °£
| µ—È–Ú∫≈ | µ—ȃ⁄»ð | µ—ÈΩ·π˚ |
| a | º”AgNO3»Ð“∫ | ”–∞◊…´≥¡µÌ…˙≥… |
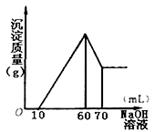
| b | º”◊„¡øNaOH»Ð“∫≤¢º”»» | ’ºØµΩ∆¯ÃÂ1.12L£®“—’€À„≥…±Í◊º ◊¥øˆœ¬µƒÃª˝£© |
| c | º”◊„¡øBaC12»Ð“∫ ±£¨∂‘À˘µ√≥¡µÌΩ¯––œ¥µ”°¢∏…‘Ô°¢≥∆¡ø£ª‘ŸœÚ≥¡µÌ÷–º”◊„¡øœ°—ŒÀ·£¨»ª∫Û∏…‘Ô°¢≥∆¡ø | µ⁄“ª¥Œ≥∆¡ø∂¡ ˝Œ™6. 27g£¨µ⁄∂˛¥Œ ≥∆¡ø∂¡ ˝Œ™2.33g |
£®1£©∏√ªÏ∫œŒÔ÷–“ª∂®≤ª¥Ê‘⁄µƒ¿Î◊” « °£
£®2£©»Ð“∫÷–“ª∂®¥Ê‘⁄µƒ“ı¿Î◊” « °£
£®3£© ‘–¥≥ˆ µ—Èb∑¢…˙∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω °£
£®4£©≈–∂œªÏ∫œŒÔ÷– «∑ҥʑ⁄K+£ø £®ÃÓ°∞ «°±ªÚ°∞∑Ò°±£©°£
¿Ì”… « °£
£®1£©Mg2+°¢Cu2+°¢Ba2+ £®3∑÷°£¥Ì–¥µπø€∑÷£¨µ´≤ª≥ˆœ÷∏∫∑÷£©°£
£®2£©SO42-°¢CO32- £®2∑÷°£¥Ì–¥µπø€∑÷£¨µ´≤ª≥ˆœ÷∏∫∑÷£©°£
£®3£©NH4+ + OH- NH3 + H2O°££®2∑÷£©
NH3 + H2O°££®2∑÷£©
£®4£© « £®1∑÷£©
¥”Â÷–ø…»∑∂®£∫NH4+ 0.05mol£¨SO42- 0.01mol£¨CO32- 0.02mol£¨∏˘æð¿Î◊”µƒµÁ∫… ÿ∫„ø…÷™“ª∂®”–K+°££®2∑÷£©
£®2£©SO42-°¢CO32- £®2∑÷°£¥Ì–¥µπø€∑÷£¨µ´≤ª≥ˆœ÷∏∫∑÷£©°£
£®3£©NH4+ + OH-
 NH3 + H2O°££®2∑÷£©
NH3 + H2O°££®2∑÷£©£®4£© « £®1∑÷£©
¥”Â÷–ø…»∑∂®£∫NH4+ 0.05mol£¨SO42- 0.01mol£¨CO32- 0.02mol£¨∏˘æð¿Î◊”µƒµÁ∫… ÿ∫„ø…÷™“ª∂®”–K+°££®2∑÷£©
¬‘

¡∑œ∞≤·œµ¡–¥∞∏
 √˚–£øŒÃ√œµ¡–¥∞∏
√˚–£øŒÃ√œµ¡–¥∞∏
œýπÿƒø
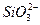 °¢
°¢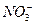
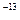 mol/Lµƒ»Ð“∫÷–£∫Fe2+°¢NH4+°¢ClO?°¢Cl?
mol/Lµƒ»Ð“∫÷–£∫Fe2+°¢NH4+°¢ClO?°¢Cl?