��Ŀ����
��10�֣���һ����������Һ��������NH4+��Fe3+��H+��Mg2+��Al3+��I����CO32�������ӣ��ֱַ�ȡ��Һ��������ʵ�飺
��1������AgNO3����Һ�л�ɫ�������ɣ�
��2��������ۣ�δ����ɫ��
��3������μ���1mol��L��1NaOH��Һ�Ĺ����У�δ�ŵ��̼������壬��NaOH��Һ�ĵ��룬��������������ͼ��ʾ��
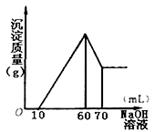
��ȷ����
��1����Һ��һ�����ڵ������� ��
��2���������������ʵ����ֱ�Ϊ ��
��1������AgNO3����Һ�л�ɫ�������ɣ�
��2��������ۣ�δ����ɫ��
��3������μ���1mol��L��1NaOH��Һ�Ĺ����У�δ�ŵ��̼������壬��NaOH��Һ�ĵ��룬��������������ͼ��ʾ��

��ȷ����
��1����Һ��һ�����ڵ������� ��
��2���������������ʵ����ֱ�Ϊ ��
��1��H+��I����Mg2+��Al3+��4�֣���һ����1�֣���һ������1�֣�����Ϊֹ�������ָ��֣���2��H+ 0.01mol ��2�֣� Al3+ 0.01mol ��2�֣� Mg2+ 0.01mol ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
