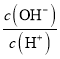��Ŀ����
����Ŀ��ijУ����С��Ϊ�ⶨij̼���ƺ�̼�����ƻ������̼���Ƶ������������ס�������ͬѧ�ֱ�������������ʵ�顣
��������ͬѧ�ó�����������������ͼ��ʾ��ʵ�����̽���ʵ�飺
��1��ʵ��ʱ�����˲����У������ձ���©���⣬��Ҫ�õ��IJ�������Ϊ____________��
��2��ϴ�ӳ���B�IJ�����__________________________________________________��
��3����ʵ���в����Ʒ����Ϊm g����������Ϊn g����̼���Ƶ���������Ϊ________��

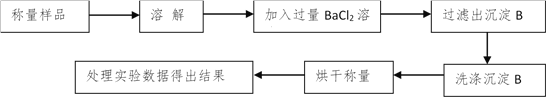
����������ͬѧ����Ҫʵ������ͼ���£�
������ͼ��ʾװ�ý���ʵ�飺
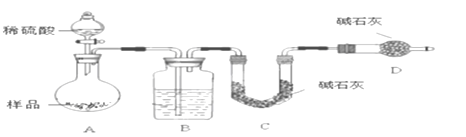
��4����ʵ����װ��Bʢ�ŵ�������_____________________����Һ©����___________����ܡ����ܡ������������ϡ�������ʵ�顣
��5����C��װ��ʯ�������վ���������塣Dװ�õ�������_________________��
��6���е�ͬѧ��ΪΪ�˼���ʵ�����ڷ�Ӧǰ��Ҫͨ��N2������ͼ����
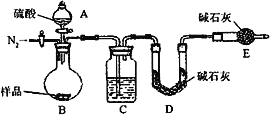
��Ӧ��ͨ��N2��Ŀ����_____________________��
���𰸡� ������ �ò�����������ע������ˮ��ֱ���պ�û����������ˮ��©���ײ���Ȼ������ظ���������2��3�� 106n/197m��100% Ũ���� ���� ���տ����е�ˮ������CO2����ȷ��ǰһ���������������������ȷ�� ��A��Bװ���в���CO2ȫ������Cװ�õļ�ʯ���У���С�������
����������������1�����˲��õ�������������̨��©�����ձ����������ȣ��������ڲ��������У�©�����ձ�����������
��2��ϴ�ӳ���Ҫע�ⲻ�ܳ�ϴ�������ò�����������ע������ˮ��ֱ���պ�û����������ˮ��©���ײ���Ȼ������ظ���������2~3����
��3������ΪBaCO3�����ݹ�ϵʽ��![]() ��̼���Ƶ���������Ϊ��
��̼���Ƶ���������Ϊ�� ![]() n g��m g��100%=
n g��m g��100%=![]() ��100%��
��100%��
����������ʵ�����̡�װ��ͼ��ʵ��Ŀ�Ŀɵã�A�в���CO2��H2O��B����ˮ����װ�ã�C�м�ʯ������A������CO2��D�м�ʯ����������������������ͨ������C���������صķ������A������CO2���������Ӷ�̼���Ƶ�����������
��4��B����ˮ����װ�ã���Bʢ�ŵ�Һ����Ũ�����Һ©���в������������ϡ�������ʵ�飬��Ϊ�����ǻӷ����ᣬ������ʹC��������HCl����������ƫ��
��5��D��ʢ�м�ʯ�ң������տ����е�ˮ������CO2���Ա�֤C��������������ȷ����
��6��ʵ�����ʱ��װ�����Դ���CO2���壬��Ӧ��ͨ��N2�ɽ�A��Bװ���в�����CO2ȫ������Cװ�õļ�ʯ���У���С���������

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�������й����ʵķ�����ȷ����
���������� | һԪ�� | ǿ����� | ��ɢϵ | |
A | Mn2O7 | ���� | ���������� | �ƺ�ˮ |
B | NO2 | ������ | ���� | ��ɫ���� |
C | SiO2 | ʯ̿�� | �������� | �ơ��� |
D | SO2 | ���� | �廯�� | ��ˮ����� |
A. AB. BC. CD. D