��Ŀ����
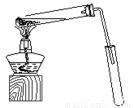
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�档�����Ҫ����գ�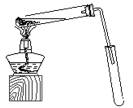
��д��ʵ�����ñ��������ˮ�Ҵ������������Ļ�ѧ����ʽ��
__________________ ��
��Ϊʹ��Ӧ���ַ�Ӧ�����´�ʩ����ȷ���� ����д��Ӧ��ţ���
����С�����ȣ���������������״̬ ���ȴ�����������״̬�����������ȱ��ַ���״̬ ��ʹ��ϡ���������� ������Ũ����������
������������ϵĵ��ܶ�һЩ���������������ռ��к�Ӱ�죬����ԭ��
�� ��
��Aͬѧ���ռ����������������뺬��������̪��NaOH��Һ�в���ˮԡ�����ȣ�������Һ�ĺ�ɫ��dz���ɴ˵ó����������к�������Ľ��ۣ�����Ϊ��һ������ȷ��Ϊʲô��
�� ��
��Bͬѧ���ռ����������������뱥��NaHCO3��Һ�У��۲쵽���������ݲ������ɵó��Ľ����� ���ù����з�����Ӧ�Ļ�ѧ����ʽ�� ��
��Cͬѧ���ռ��������������������뱥��Na2CO3��Һ�У������ݲ��������ǵó������������в�������Ľ��ۡ�����������ѧ֪ʶ�����۸�ͬѧ�Ľ����Ƿ���ȷ��
�ҵ������ǣ� ��
��CH3COOH+C2H5OH CH3COOC2H5+H2O
CH3COOC2H5+H2O
�Ƣ٢�
��ʹ���������ò�����ֵ�������ʹ�ռ������٣�����Ҫ�����ȷ������õ����������
�Ȳ���ȷ�������������ڼ��������»ᷢ��ˮ�⡣���ɵ�����Ҳ���к�NaOH�Ӷ�ʹ��̪��ɫ���ʷ�̪��ɫ���ܿ϶��Ǻ���������ɵġ�
����������������� CH3COOH+NaHCO3=CH3COONa+CO2��+H2O
�ʸý��۲���ȷ���������Na2CO3��Ӧ��������NaHCO3�����ų����ݣ���Na2CO3ȫ��ת��ΪNaHCO3���������NaHCO3��Ӧ��������(CO2)�����ԣ�û�����ݲ�����������˵�����в���������
����