��Ŀ����
��08���Ϻ�������֪�� ����A����������ø�������»�����Ϊ�к�����GHB����ͼ�ǹ�������A��һ���Ʊ���������A������һϵ�л�ѧ��Ӧ��
![]()

��ش��������⣺
(1��д����Ӧ���ͣ���Ӧ��____________����Ӧ��____________��
(2��д��������B�Ľṹ��ʽ_____________________________��
(3��д����Ӧ�ڵĻ�ѧ����ʽ____________________________��
(4��д����Ӧ�ܵĻ�ѧ����ʽ____________________________��
(5����Ӧ���г�����E�⣬�����ܴ���һ�ָ�����(��![]() �ṹ)�����Ľṹ��ʽΪ________________��
�ṹ)�����Ľṹ��ʽΪ________________��
(6���뻯����E��Ϊͬ���칹������ʲ�����Ϊ________����д��ĸ����
a���� b��ȩ c������ d����
����
��1���ӳɷ�Ӧ����ȥ��Ӧ��
��2��HOCH2CH2CH2CHO��
��3��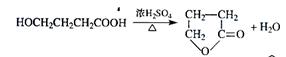
��4��
��5��
��6��d��
����������HOCH2C![]() CCH2OH��H2��Ӧ�ɵã�AΪHOCH2CH2CH2CH2OH��Aת��ΪD��������Ũ���Ტ���ȣ�����������ȥ��Ӧ������DΪ1��3������ϩ��Dת��ΪC7H10O2����C4H6��CH2=CH��COOH���Կ�����Dת��ΪEΪ�ӳɷ�Ӧ����GHBת��ΪC��֪��GHB���У�OH�ͣ�COOH������GHB�Ľṹ��ʽΪ��HO��CH2CH2CH2��COOH������ΪBΪHO��CH2CH2CH2��CHO��GHBת��ΪC�Ƿ����ڷ���������Ӧ����������C7H10O2�IJ����Ͷ�Ϊ
CCH2OH��H2��Ӧ�ɵã�AΪHOCH2CH2CH2CH2OH��Aת��ΪD��������Ũ���Ტ���ȣ�����������ȥ��Ӧ������DΪ1��3������ϩ��Dת��ΪC7H10O2����C4H6��CH2=CH��COOH���Կ�����Dת��ΪEΪ�ӳɷ�Ӧ����GHBת��ΪC��֪��GHB���У�OH�ͣ�COOH������GHB�Ľṹ��ʽΪ��HO��CH2CH2CH2��COOH������ΪBΪHO��CH2CH2CH2��CHO��GHBת��ΪC�Ƿ����ڷ���������Ӧ����������C7H10O2�IJ����Ͷ�Ϊ![]() =3��a�� b��c���������뻯����E��Ϊͬ���칹�壬����Ƿ��࣬�غ������������IJ����Ͷ�
=3��a�� b��c���������뻯����E��Ϊͬ���칹�壬����Ƿ��࣬�غ������������IJ����Ͷ�![]() =
=![]() =4�����Բ������Ƿ��ࡣ
=4�����Բ������Ƿ��ࡣ

 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д�![]()
ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
1 | 10mLFeSO4��Һ | 10mLNH3 | ���ɰ�ɫ���������ɫ |
2 | 20mLH2S | 10mLSO2 |
|
3 | 30mLNO2(��Ҫ) | 10mLH2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
4 | 15molCl2 | 40mLNH3 |
|
(1��ʵ��1�У��������ձ�Ϊ________ɫ��д��������ɫ�Ļ�ѧ����ʽ________________________��
(2��ʵ��2����Ͳ�ڵ������ǣ���___________���ɣ�����________�ƶ��������⡢���ڡ���������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
(3��ʵ��3�У����е�3mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ_______________________________��
(4��ʵ��4�У���֪��3Cl2��2NH3��N2��6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ��Ϊ____________�������Ͳ��ʣ����������ԼΪ________mL��