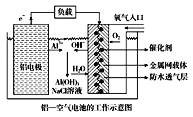
��Ŀ����
����Ŀ��ij��ʵ������450mL��![]() ��H2SO4����Һ����ʵ����������������Ϊ98%���ܶ�Ϊ1.84g/ml��Ũ���ᡣ
��H2SO4����Һ����ʵ����������������Ϊ98%���ܶ�Ϊ1.84g/ml��Ũ���ᡣ
�밴Ҫ��ش��������⣺
��1��ʵ����Ҫ�IJ������������ձ�����ͷ�ιܡ�������������______��
��2�����㣺�����ŨH2SO4�����Ϊ_____ml��
��3�����ƹ��̣�
![]() ȷ��ȡһ�������ŨH2SO4
ȷ��ȡһ�������ŨH2SO4
![]() ��ŨH2SO4���ձ��ڻ���ע���ձ��У����������ϡ��
��ŨH2SO4���ձ��ڻ���ע���ձ��У����������ϡ��
![]() ���ձ��е���Һ��ȴ���ò���������ת�Ƶ��Ѿ���©�ĺ��ʹ�������ƿ��
���ձ��е���Һ��ȴ���ò���������ת�Ƶ��Ѿ���©�ĺ��ʹ�������ƿ��
![]() ������ƿ�м�������ˮ���ݣ��ھ���̶���
������ƿ�м�������ˮ���ݣ��ھ���̶���![]() ʱ�����ý�ͷ�ιܼ�����ˮ���̶���
ʱ�����ý�ͷ�ιܼ�����ˮ���̶���
![]() �Ǻ�ƿ�����������µߵ���ҡ��
�Ǻ�ƿ�����������µߵ���ҡ��
![]() �����ƺõ���Һת�����Լ�ƿ�д��á�
�����ƺõ���Һת�����Լ�ƿ�д��á�
����������ȱ��_______________________��Ӧ���ڲ���_____֮ǰ![]() ���š�
���š�![]() ��
��
��4���ں�������д���и����������������ҺŨ�ȵ�Ӱ��![]() ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족
ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족![]() ��
��
![]() ��ȡŨH2SO4���ʱ�����ӿ̶���_______��
��ȡŨH2SO4���ʱ�����ӿ̶���_______��
![]() ����ʱ������Һ��______��
����ʱ������Һ��______��
![]() ����ƿ����������ˮ________��
����ƿ����������ˮ________��
�����ձ���ϡ����Ũ��������ת��������ƿ�м�ˮ����_______��
�ݶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�ˮ���̶���_______��
��5�����в����У�����ƿ�����߱��Ĺ�����______________![]() ��ѡ
��ѡ![]() ��
��
A.����һ�����ȷŨ�ȵı���Һ
B.������Һ
C.��������ƿ������µ����������Һ��
D.ȷϡ��ijһŨ�ȵ���Һ
E.���������ܽ��������
���𰸡���Ͳ��500mL����ƿ 13.6 ϴ���ձ��Ͳ���������ϴ��Һһ��ע������ƿ�� �� ƫ�� ƫ�� ��Ӱ�� ƫ�� ƫ�� B��C��D��E
��������
����������Һ��ԭ�������������������������裬���������ܵ�ʵ����
(1)��ȡһ�����Ũ������Ҫ��Ͳ����ͷ�ιܣ�ϡ��Ũ������Ҫ�ձ���������������450mL��Һ��Ҫ500mL����ƿ��ʵ����Ҫ�IJ������������ձ�����ͷ�ιܡ�����������Ҫ��Ͳ��500mL����ƿ��
(2)����ŨH2SO4�����ΪV����ϡ��ǰ���������������1.84g/mL��V��98%��0.500L��![]() ��98g/mol�����V��13.6mL��
��98g/mol�����V��13.6mL��
(3)�������ƹ��̿�֪���ڲ���![]() ֮����Ҫ��������ˮϴ���ձ��Ͳ�����2~3�Σ�����ϴ��Һһ��ע������ƿ����
֮����Ҫ��������ˮϴ���ձ��Ͳ�����2~3�Σ�����ϴ��Һһ��ע������ƿ����
(4)��c=n/V������n��V��c��Ӱ�졣
![]() ��ȡŨH2SO4ʱ���ӿ̶��ߣ���Ũ�������ƫС��nƫС��cƫ����
��ȡŨH2SO4ʱ���ӿ̶��ߣ���Ũ�������ƫС��nƫС��cƫ����
![]() ����ʱ����Һ�棬��VƫС��cƫ����
����ʱ����Һ�棬��VƫС��cƫ����
![]() ������Һ�����л���������ƿ�м�ˮ��������ƿ����������ˮ��c��Ӱ����
������Һ�����л���������ƿ�м�ˮ��������ƿ����������ˮ��c��Ӱ����
����ϡ�͵�Ũ�������ȵģ�����ת�Ʋ���ˮ���ݡ��Ժ���Һ��Ȼ��ȴ��ʹVƫС��cƫ����
������ҡ��ʱ����������Һ�����ڿ̶����Ϸ��ڱ��ϣ�ʹҺ����ڿ̶��ߡ���������������Ҫ�������ټ�ˮ���̶��ߣ���ʹVƫ��cƫ�͡�
(5)����ƿֻ����������һ�����ȷ���ʵ���Ũ�ȵ���Һ������������Һ��B�������������ƿֻ��һ���̶��ߣ����ܲ���Һ�������C�������������ƿ�̶��������ݻ���ȷ���������Ȼ���ȴ��ϡ���̰��������仯��DE����ѡB��C��D��E��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ijͬѧ������װ�ý���ʵ��������������ͬʱ���ڣ���¼�������£���Һ���¶ȱ仯�������ԣ���
ʵ��װ�� | ʵ����� | �缫���� | ʵ������ |
| �� | �� | ����������������ɫ������ ����������Һ��δ����ɫ���� |
�� | ʯī | ����������������ɫ������ ������δ����ɫ������ ������������ɫ������������ð�ɫ���壬�����ܽ⣬�������� |
����ʵ����������˵����ȷ����
A. �٢��У������ĵ缫��Ӧʽ��2H2O��4e == O2��+ 4H+
B. ���У���ɫ���ǵ���Ҫ�ɷ���Ca(OH)2
C. ���У�������ɫ���ǵ���Ҫԭ���ǵ���������ˮ
D. ���У�������ɫ�������������ϱ���������CO32�й�
����Ŀ��Ϊ�о���������������֮��ķ�Ӧ��ij�о�С�����ÿ�Ѩ�������ʵ��̽����
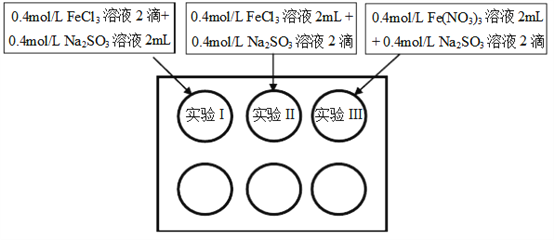
С���¼��ʵ���������±���ʾ��
��ʼʱ | 5min�� | 3��� | |
ʵ��I | ��Һ������Ϊ���ɫ����II��III����dz | �뿪ʼ���ʱһ�� | ��Һ�ʻ�ɫ���ײ����ֺ��ɫ���� |
ʵ��II | ��Һ������Ϊ���ɫ | ���ɫ���Ա�dz | ��Һ�ʻ���ɫ |
ʵ��III | ��Һ������Ϊ���ɫ | ���ɫ��dz����II���� | ��Һ�ʻ�ɫ |
��1�����ʵ������0.4mol/L FeCl3��Һ��Fe(NO3)3��ҺpH��ԼΪ1.0������Һ�������Ե�ԭ����__________�������ӷ���ʽ��ʾ����
��2����ʼ���ʱ��ʵ��I�к��ɫ��II��III����dz��ԭ����___________��
��3��Ϊ��̽��5min��ʵ��II��III����Һ���ɫ��dz��ԭ��С��ͬѧ�����ʵ��IV���ֱ�ȡ����5min��ʵ��I��II��III����Һ������2�����軯����Һ������ʵ��II��III�г�����ɫ������ʵ��I�������Ա仯������ʵ��IV������ϻ�ѧ������ͺ��ɫ��dz��ԭ����___________��
��4�����5min��ʵ��III����Һ��ɫ��ʵ��II���С��ͬѧ��Ϊ���ܴ����������أ�
��Cl- ���Լӿ�Fe3+��SO32-��������ԭ��Ӧ��
��___________��
��NO3- �����Ի����´���Fe3+������SO32-��ͬʱ����H+��ʹFe3+ˮ�����Fe(OH)3�϶ࡣ
ͨ��ʵ��V��ʵ��VI��������̽����
ʵ���������֪Na+��ʵ����Ӱ�죩 | 5min������� | |
ʵ��V | ��2mL pH=1.0��0.4mol/L Fe(NO3)3��Һ���ܽ�Լ___________���壬�ټ���2��0.4mol/L Na2SO3��Һ | ��Һ�ĺ��ɫ����II��III֮�� |
ʵ��VI | ��2mL pH=1.0��ϡ�������ܽ�Լ0.19g NaNO3���壬�ټ���2��0.4mol/L Na2SO3��Һ�������е�������BaCl2��Һ | _____________ |
ʵ����ۣ����آٺ����آھ����������آ۲����ԡ��뽫����������д������
��5��ͨ������ʵ�飬���½�������ۺ�������___________������ĸ����
a��Fe3+��SO32-ͬʱ����ˮ�ⷴӦ��������ԭ��Ӧ����ˮ�ⷴӦ�����ʿ죬�ȴ��㹻��ʱ�����������ԭ��ӦΪ��
b��Ũ��Ϊ1.2 mol/L��ϡ������5min�ڲ��ܽ�Fe2+����
c����pH����1��ϡ�����м�������Ba(NO3)2��ʹ����ȫ�ܽ⣬����������ʵ��I���ϲ���Һ���Ƿ����SO42-