��Ŀ����
���ǵؿ��к�������Ԫ�ء�
��1����Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�����Ϊ ��.
��2��H2O�����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ ��

��3�� H+����H2O�γ�H3O+��H3O+��Oԭ�Ӳ��� �ӻ���H3O+��H-O-H���DZ�H2O��H-O-H���Ǵ�ԭ��Ϊ ��
(4)CaO��NaCl�ľ���ͬΪ���������ṹ����֪CaO�����ܶ�Ϊag cm-3,
cm-3, ��ʾ�����ӵ���������CaO�������Ϊ Cm3��
��ʾ�����ӵ���������CaO�������Ϊ Cm3��
��1����Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�����Ϊ ��.
��2��H2O�����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ ��

��3�� H+����H2O�γ�H3O+��H3O+��Oԭ�Ӳ��� �ӻ���H3O+��H-O-H���DZ�H2O��H-O-H���Ǵ�ԭ��Ϊ ��
(4)CaO��NaCl�ľ���ͬΪ���������ṹ����֪CaO�����ܶ�Ϊag
 cm-3,
cm-3, ��ʾ�����ӵ���������CaO�������Ϊ Cm3��
��ʾ�����ӵ���������CaO�������Ϊ Cm3��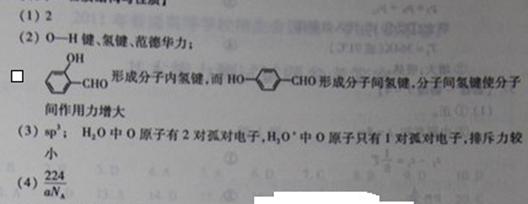
��

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ