��Ŀ����
9����1��Ҫ����1.84mol/L������100mL��������Ͳ���ձ���������֮�⣬����Ҫ�õ��IJ���������100mL����ƿ����ͷ�ιܣ���2�����ݻ�ѧ���ʵ�����д�����ǵĻ�ѧʽ���ٵ���CuSO4•5H2O������KAl��SO4��2•12H2O
��3����ijһ��Һ�к���Fe2+��Fe3+��д������Fe2+�Ĵ��ڵ�ʵ�������ȡ��Һ���Թ��У��μ����Ը��������Һ������ɫ��ȥ��֤����Fe2+����
���� ��1���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2��CuSO4•5H2O�׳Ƶ�����KAl��SO4��2•12H2O���������׳ƣ�
��3������������ʹ���Ը��������Һ��ɫ
��� �⣺��1���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪���������Ϊ����Ͳ���ձ�����������100 mL����ƿ�ͽ�ͷ�ιܣ��ʻ���Ҫ�õ�����100 mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��100 mL����ƿ����ͷ�ιܣ�
��2��CuSO4•5H2O�׳Ƶ������������Ļ�ѧʽΪCuSO4•5H2O��KAl��SO4��2•12H2O���������׳ƣ��������Ļ�ѧʽΪ��KAl��SO4��2•12H2O���ʴ�Ϊ��CuSO4•5H2O��KAl��SO4��2•12H2O��
��3�����Ը��������ǿ�����ԣ����������������ʹ����ɫ�����Լ����������ӵķ����ǣ�ȡA����Һ���μ����Ը��������Һ������ɫ��ȥ��֤����Fe2+��
�ʴ�Ϊ��ȡ��Һ���Թ��У��μ����Ը��������Һ������ɫ��ȥ��֤����Fe2+��
���� ���⿼������Һ���ƹ�����������ѡ������ʵ��׳Ƶ����⣬Ӧע������Fe2+��Fe3+�ļ��飮

��ϰ��ϵ�д�
�����Ŀ
19���������ʣ������� �ڶ������� �ۻ���̿ ��Ư�ۣ�����ʹƷ����Һ��ɫ������ɫ���̲�����������ԭ��Ӧ���ǣ�������
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢۢ� |
20������˵������ȷ���ǣ�������
| A�� | ��ͨ�����Ը��������Һ�������������Ȳ | |
| B�� | ��ϩ��ʯ���ѽ��IJ��� | |
| C�� | ʯ�͵ķ���ú�ĸ����������仯��ʯ�͵��ѽ���ѻ��ǻ�ѧ�仯 | |
| D�� | ���������ʡ����۶��Ǹ߷��ӻ�������ܷ���ˮ�ⷴӦ |
17�����б仯���ʩ������������ԭ�����͵��ǣ�������
| A�� | ����ɫ��NO2��ѹ����ɫ�ȱ����ٱ�dz | |
| B�� | H2��I2��HI��������ѹ����ɫ���� | |
| C�� | �ϳɰ�ʱ���¡���ѹ�Ժϳɰ����� | |
| D�� | ������Һϡ��ʱ����ҺpH���� |
4�������£��������һԪ��BOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH���±���ʾ��
����ʵ�������ش��������⣺
��1����д��BOH����Һ�еĵ��뷽��ʽ��BOH?B++OH-
��2����������Һ������Ũ���ɴ�С��˳��Ϊ��c��Cl-����c��B+����c��H+����c��OH-��
��3����������Һ��B-����Ũ��c��B-��=0.05 mol/L
��4����������Һ��c��BOH����c��B-����c��Cl-��Ũ�ȴ�С��ϵΪc��BOH����c��B+����c��Cl-��
��5����������ʵ�飬��ʽ������BOH�ڳ����µĵ���ƽ�ⳣ��Kb=5��10-8��
| ʵ����� | HCl��ҺŨ�ȣ�mol/L�� | BOH��Һ��Һ��mol/L�� | ��Ϻ���ҺpH |
| �� | 0.10 | 0.10 | 4.7 |
| �� | 0.10 | 0.30 | 7 |
| �� | 0.10 | 0.50 | 8.2 |
��1����д��BOH����Һ�еĵ��뷽��ʽ��BOH?B++OH-
��2����������Һ������Ũ���ɴ�С��˳��Ϊ��c��Cl-����c��B+����c��H+����c��OH-��
��3����������Һ��B-����Ũ��c��B-��=0.05 mol/L
��4����������Һ��c��BOH����c��B-����c��Cl-��Ũ�ȴ�С��ϵΪc��BOH����c��B+����c��Cl-��
��5����������ʵ�飬��ʽ������BOH�ڳ����µĵ���ƽ�ⳣ��Kb=5��10-8��
14�����и�������������ķ�����һ����ȵ��ǣ�������
| A�� | �¶���ͬ�������ͬ��O2��N2 | B�� | ������ȡ��ܶȲ��ȵ�N2��C2H4 | ||
| C�� | �����ͬ���ܶȲ��ȵ�CO��C2H4 | D�� | ѹǿ��ͬ�������ͬ��O2��H2 |
1��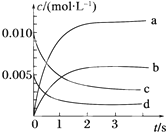 ��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��1��������Ӧ�ǣ���ǡ����ǡ������淴Ӧ���ڵ�5sʱ��NO��ת����Ϊ65%����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v=0.0015mol/��L•s����
��2����ͼ��ʾ����ʾNO2�仯���ߵ���b��
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����bc������ţ���
a��v��NO2��=2v��O2�� b��������ѹǿ���ֲ���
c��v����NO��=2v����O2�� d���������ܶȱ��ֲ��䣮
 ��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO��/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��2����ͼ��ʾ����ʾNO2�仯���ߵ���b��
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����bc������ţ���
a��v��NO2��=2v��O2�� b��������ѹǿ���ֲ���
c��v����NO��=2v����O2�� d���������ܶȱ��ֲ��䣮
16������������ͭ���ʪ��ұ���Ѿ�ȡ�úܴ�Ľ�չ������һ�ִ����������ͭ���ʪ��ұ�������乤��������1ͼ��ʾ��

�¶ȡ���Һ�ȡ���Ӧʱ�䡢������Ũ�ȶ���ͭ�Ľ������нϴ��Ӱ�죬������ʵ��ó����⼸�����ض�ͭ�Ľ�����Ӱ��ı仯����ͼ����ͼ2-ͼ5��ʾ��
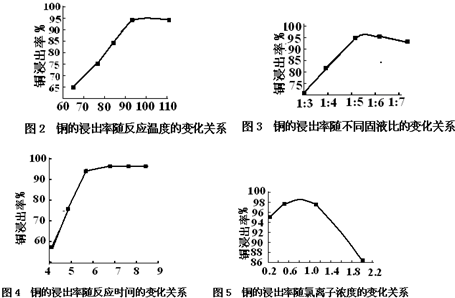
��1������ʵ���Լ���ҵ������ʵ��Ҫ��ͼ2-ͼ5�еó�����ѹ�������Ϊ�����±���ѡ����ţ�A��
��2���������̴���Һ�еõ�������FeSO4•7H2O����IJ���������Ũ������ȴ�ᾧ�����ˡ��ñ�ˮϴ�ӡ����Ҵ���ϴ��������Ҵ���ϴ��Ŀ���ǣ������Ҵ��ӷ������������
��3������ƷFeSO4•7H2O��Ʒ�Ĵ��ȿ��õζ������вⶨ��ʵ�鲽�����£�
����1����ȡ5.800g�̷���Ʒ�����ܽ⡢���ݵȲ���ȷ����250mL��Һ��
����2������������ƿ����ȡ25.00mL������Һ����ƿ�У�
����3���������ữ��0.0100mol/L KMnO4��Һ�ζ����յ㣬��¼����KMnO4��Һ���
����4���ظ�����2������3һ�����Σ�
�ٲ���1���õ��IJ��������У��ձ�������������ͷ�ιܺ�250mL����ƿ������2��ȡ��Һ������������ʽ�ζ��ܣ�
��д������3��Ӧ�����ӷ���ʽ5Fe2++MnO-4+8H+=5Fe3++Mn2++4H2O��
�����ݴ�����
����������Ʒ��FeSO4•7H2O����������Ϊ95.86%��
�ܲ����Dz����������������ⶨ����Ʒ��FeSO4•7H2O����������ƫ�ͣ���ƫ�͡�ƫ����Ӱ�죩��

�¶ȡ���Һ�ȡ���Ӧʱ�䡢������Ũ�ȶ���ͭ�Ľ������нϴ��Ӱ�죬������ʵ��ó����⼸�����ض�ͭ�Ľ�����Ӱ��ı仯����ͼ����ͼ2-ͼ5��ʾ��

��1������ʵ���Լ���ҵ������ʵ��Ҫ��ͼ2-ͼ5�еó�����ѹ�������Ϊ�����±���ѡ����ţ�A��
| ��Ӧ�¶�/�� | ��Һ�� | c��Cl-��/mol•L-1 | ��Ӧʱ��/h | |
| A | 95 | 1��5.5 | 0.8 | 6 |
| B | 100 | 1��5.5 | 0.7 | 7 |
| C | 110 | 1��6 | 0.9 | 8 |
��3������ƷFeSO4•7H2O��Ʒ�Ĵ��ȿ��õζ������вⶨ��ʵ�鲽�����£�
����1����ȡ5.800g�̷���Ʒ�����ܽ⡢���ݵȲ���ȷ����250mL��Һ��
����2������������ƿ����ȡ25.00mL������Һ����ƿ�У�
����3���������ữ��0.0100mol/L KMnO4��Һ�ζ����յ㣬��¼����KMnO4��Һ���
����4���ظ�����2������3һ�����Σ�
�ٲ���1���õ��IJ��������У��ձ�������������ͷ�ιܺ�250mL����ƿ������2��ȡ��Һ������������ʽ�ζ��ܣ�
��д������3��Ӧ�����ӷ���ʽ5Fe2++MnO-4+8H+=5Fe3++Mn2++4H2O��
�����ݴ�����
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00mL | 0.02 | 40.01 |
| 2 | 25.00mL | 0.70 | 40.71 |
| 3 | 25.00mL | 0.20 | 39.20 |
�ܲ����Dz����������������ⶨ����Ʒ��FeSO4•7H2O����������ƫ�ͣ���ƫ�͡�ƫ����Ӱ�죩��
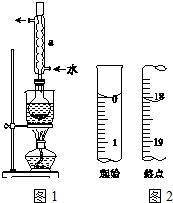 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���