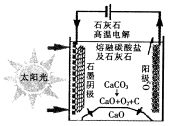
��Ŀ����
���ǵ�ѭ�������е���Ҫ����,���ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
(1)����ͼ1�ṩ����Ϣ,д���÷�Ӧ���Ȼ�ѧ����ʽ:��������������������������������������������,��ͼ1��������������������(�a����b��)��ʾ��������ý�������仯���ߡ� 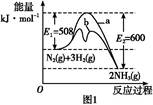
(2)�ں���������,������������˵��������Ӧ�Ѵ�ƽ���������������
A.3v(H2)��=2v(NH3)��
B.��λʱ��������n mol N2��ͬʱ����2n mol NH3
C.���������ܶȲ��ٸı�
D.������ѹǿ����ʱ��ı仯���仯
(3)һ���¶���,��2 L�ܱ������г���1 mol N2��3 mol H2,�����������,0.5 min��ﵽƽ��,�����������0.4 mol NH3,��ƽ����Ӧ����v(N2)=������������,���¶��µ�ƽ�ⳣ��K=�����������������������¶�,Kֵ�仯��������(���������С�����䡱)��
(4)Ϊ��Ѱ�Һϳ�NH3���¶Ⱥ�ѹǿ����������,ijͬѧ���������ʵ��,����ʵ�������Ѿ���������ʵ����Ʊ��С�
| ʵ���� | T(��) | n(N2)/n(H2) | p(MPa) |
| �� | 450 | 1/3 | 1 |
| �� | �� | �� | 10 |
| �� | 480 | �� | 10 |
A.�����ϱ��ո�������ʣ���ʵ���������ݡ�
B.���ݷ�ӦN2(g)+3H2(g)
 2NH3(g)���ص�,�ڸ���������ͼ2��,��������1 MPa��10 MPa������H2��ת�������¶ȱ仯����������ʾ��ͼ,�������������ߵ�ѹǿ��
2NH3(g)���ص�,�ڸ���������ͼ2��,��������1 MPa��10 MPa������H2��ת�������¶ȱ仯����������ʾ��ͼ,�������������ߵ�ѹǿ��
(1)N2(g)+3H2(g) 2NH3(g)��H="-92" kJ��mol-1��b
2NH3(g)��H="-92" kJ��mol-1��b
(2)BD��(3)0.2 mol��L-1��min-1��0.058����С
(4)A.��.450��1/3����.1/3
B.
����

�״�ȼ�Ϸ�Ϊ�״����ͺͼ״����͡���ҵ�Ϻϳɼ״��ķ����ܶࡣ
��1��һ�������·�����Ӧ��
��CO2��g�� +3H2��g�� ��CH3OH��g��+H2O��g�� ��H1
��2CO��g�� +O2��g�� ��2CO2��g�� ��H2
��2H2��g��+O2��g�� ��2H2O��g�� ��H3
��CO��g�� + 2H2��g��  CH3OH��g�����ġ�H�� ��
CH3OH��g�����ġ�H�� ��
��2�����ݻ�Ϊ2L���ܱ������н��з�Ӧ�� CO��g��+2H2��g�� CH3OH��g�� �������������䣬��300���500��ʱ�����ʵ���n��CH3OH�� �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����
CH3OH��g�� �������������䣬��300���500��ʱ�����ʵ���n��CH3OH�� �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����
��3����Ҫ��״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____________������ĸ����
| A����������� |
| B�������¶� |
| C�������¶� |
| D��ʹ�ú��ʵĴ��� |
��4��CH4��H2O�ڴ������淢����ӦCH4+H2O
 CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣���
CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣�����5���Լ״�Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺

��B���ĵ缫��ӦʽΪ ��
�����ø�ȼ�ϵ������Դ����ʯī���缫�������ͭ��Һ������·��ת��1mole- ʱ��ʵ�������ĵļ״��������������ϴ���ԭ���� ��
��6��25��ʱ������Ƶ�Ksp=4.0��10-8,̼��Ƶ�Ksp=2.5��10-9����20ml̼��Ƶı�����Һ����μ���8.0��10-4 mol��L-1�IJ������Һ20ml���ܷ�������� ����ܡ�����
�µġ���������������������2016��1��1�����ҹ�ȫ��ʵʩ���ݴˣ�������������ָ��(AQI)�ձ���ʵʱ���������SO2��NO2��CO��O3��PM10��PM2.5��ָ�꣬Ϊ�����ṩ����ָ�����������ؾ���������ų��к����
(1)�����ų���β���к���CO��NO�����壬�û�ѧ����ʽ���Ͳ���NO��ԭ��________________________________________
(2)�����������ڰ�װ�Ĵ�ת��������ʹ����β���е���Ҫ��Ⱦ��ת��Ϊ���Ĵ���ѭ�����ʡ���֪��
N2(g)��O2(g)===2NO(g)����H����180.5 kJ��mol��1
2C(s)��O2(g)===2CO(g)����H����221.0 kJ��mol��1
C(s)��O2(g)===CO2(g)����H����393.5 kJ��mol��1
��Ӧ2NO(g)��2CO(g)??N2(g)��2CO2(g)�Ħ�H��________kJ��mol��1���÷�Ӧ�Ħ�S________0(�>����<������)��
(3)��0.20 mol NO��0.10 mol CO����һ���ݻ��㶨Ϊ1 L���ܱ������У��ڲ�ͬ�����·�Ӧ�����в������ʵ�Ũ�ȱ仯״����ͼ��ʾ��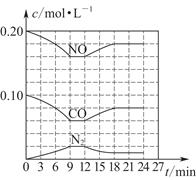
�ټ������N2��6��9 minʱ��ƽ����Ӧ����v(N2)��________mol��L��1��min��1��
�ڵ�12 minʱ�ı�ķ�Ӧ����Ϊ________(����¡����¡�)��
�ۼ��㷴Ӧ�ڵ�24 minʱ��ƽ�ⳣ��K��________���������¶Ȳ��䣬���������г���CO��N2��0.060 mol��ƽ�⽫________�ƶ�(�����������)��
(4)��������л����ó������ⶨ�����к��нϸ�Ũ��SO2�ĺ����������һЩ������20 ����������±���
| �ܽ��(S)/g | �ܶȻ�(Ksp) | ||
| Ca(OH)2 | Ba(OH)2 | CaSO3 | BaSO3 |
| 0.160 | 3.89 | 6.76��10��3 | 5.48��10��9 |
������SO2����ʵ��Լ���________[�Ca(OH)2����Ba(OH)2��]��Һ��
����20 ��ʱ����CaSO3����Һ�еμ�������BaCl2��Һ����CaSO3��BaSO3��ת���ﵽƽ��ʱ����Һ�е�
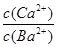 ��____________(д������ʽ����)��
��____________(д������ʽ����)�� ��¯������ұ��������Ҫ��������������Ҫ��ӦΪ��Fe2O3��s����3CO��g��??2Fe��s����3CO2��g��
��H��a kJ��mol��1��
��1����֪����Fe2O3��s����3C��s��ʯī��=2Fe��s����3CO��g��
��H1����489.0 kJ��mol��1��
��C��s��ʯī����CO2��g��=2CO��g������H2����172.5 kJ��mol��1����a��________��
��2��ұ������Ӧ��ƽ�ⳣ������ʽK��________���¶����ߺ�Kֵ________������������䡱��С������
��3����T ��ʱ���÷�Ӧ��ƽ�ⳣ��K��64����2 L�����ܱ����������У��ֱ��±���ʾ�������ʣ���Ӧ����һ��ʱ���ﵽƽ�⡣
| | Fe2O3 | CO | Fe | CO2 |
| ��/mol | 1.0 | 1.0 | 1.0 | 1.0 |
| ��/mol | 1.0 | 2.0 | 1.0 | 1.0 |
�ټ�������CO��ƽ��ת����Ϊ________��
������˵����ȷ����________������ĸ����
a���������������ܶȺ㶨ʱ����־��Ӧ�ﵽƽ��״̬
b������Fe2O3�������������CO��ת����
c����������CO��ƽ��ת���ʴ����ҵ�ƽ��ת����
d���ס��������У�CO��ƽ��Ũ��֮��Ϊ2��3
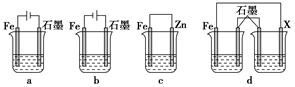
��4����ȡһ����ʩ�ɷ�ֹ������ʴ������װ���е��ձ����ʢ�е�Ũ�ȡ��������NaCl��Һ��
����a��b��cװ�����ܱ���������________������ĸ����
������dװ�ñ�������X���ĵ缫����Ӧ��________�������ƣ���
��5��25 ��ʱ�й����ʵ��ܶȻ����£�Ksp[Mg��OH��2]��5.61��10��12��Ksp[Fe��OH��3]��2.64��10��39��25 ��ʱ������Mg2����Fe3������Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH��8ʱ��c��Mg2������c��Fe3������________��
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H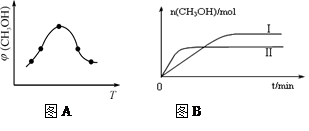
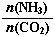 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ��
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ �� ����ش��������⣺
����ش��������⣺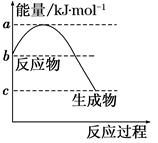
 H2NCOONH4(l)(���������)����H1
H2NCOONH4(l)(���������)����H1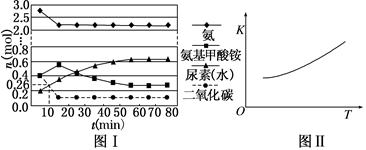
 O2(g)=FeO(s)����H����272.0 kJ��mol��1
O2(g)=FeO(s)����H����272.0 kJ��mol��1 O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1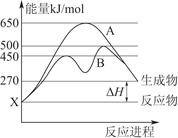
 Na2S(s)��4H2O(g)
Na2S(s)��4H2O(g)
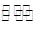 C H4 (g)+2 H2O(g)����һ�ݻ�Ϊ2 L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2 0.2 mol��Lһ1��H2 0.8 mol��Lһ1��CH40.8 mol��Lһ1��H2O1.6 mol��Lһ1����CO2��ƽ��ת����Ϊ________��300 ��ʱ������Ӧ��ƽ�ⳣ��K=____________________��200��ʱ�÷�Ӧ��ƽ�ⳣ��K=64.8����÷�Ӧ�ġ�H_____(���������<��)O��
C H4 (g)+2 H2O(g)����һ�ݻ�Ϊ2 L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2 0.2 mol��Lһ1��H2 0.8 mol��Lһ1��CH40.8 mol��Lһ1��H2O1.6 mol��Lһ1����CO2��ƽ��ת����Ϊ________��300 ��ʱ������Ӧ��ƽ�ⳣ��K=____________________��200��ʱ�÷�Ӧ��ƽ�ⳣ��K=64.8����÷�Ӧ�ġ�H_____(���������<��)O��