��Ŀ����
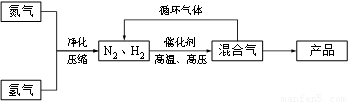
��ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��N2��g��+3H2��g��?2NH3��g�����䲿�ֹ����������£�
�ش��������⣺
��1����֪��N2��g��+O2��g��=2NO��g������H=180.5kJ/mol
4NH3��g��+5O2��g��=4NO��g��+6H2O��g������H=-905kJ/mol
2H2��g��+O2��g��=2H2O��g������H=-483.6kJ/mol
��N2��g��+3H2��g��?2NH3��g���ġ�H=______��
��2�������ҵ�ϣ���һ���¶��£���1.5molN2 �����6molH2 ����ͨ�뵽���Ϊ1�����ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%��������ת����Ϊ______���÷�Ӧ��ƽ�ⳣ������ʽΪ______���ı�������������ʹƽ��������Ӧ���������ƽ�ⳣ���������______
������ѹǿ ������Ӧ���Ũ�� ��ʹ�ô��� �ܽ����¶�
��3���������������������Ͱ����Ĺܵ��Ƿ�©�������©������а��̣��ɷ�Ϊ�Ȼ�泥����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ��______��
��4������ó������İ�ˮ��pH=a��������ͬ���������ʱ����Һ�����ԣ���������pH______14-a������ڡ���С�ڡ����ڡ���
���𰸡���������1�����ݸ�˹���ɼ��㣮
��2������ѹǿ֮�ȵ������ʵ���֮�ȼ����ƽ������������ʵ��������ò���������μӷ�Ӧ�ĵ��������ʵ���������ת���ʶ�����㵪����ת���ʣ�
ƽ�ⳣ��ָ������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��
ƽ�ⳣ��ֻ���¶�Ӱ�죬ƽ�ⳣ�����䣬�¶Ȳ��ܱ仯����Ϸ�Ӧ�ص㣬�������������ƽ���Ӱ���������жϣ�
��3����Ӧ���Ȼ�����ɣ���Ԫ�صĻ��ϼ۽��ͣ�����������ԭ��Ӧ����֪��Ԫ�ر�����Ϊ����������������ԭ��Ӧ��ƽд������ʽ��
��4����ϳ����ԣ��������ӵ�Ũ�ȵ���笠�����Ũ�ȣ�����һˮ�ϰ���������ʣ�����ˮ��Ũ�ȴ���10-14+a���������������ӵ�Ũ�ȴ��ڴ���10-14+a����������������Ũ�ȴ���10-14+a��
����⣺��1����֪����N2��g��+O2��g��=2NO��g������H=180.5kJ/mol
��4NH3��g��+5O2��g��=4NO��g��+6H2O��g������H=-905kJ/mol
��2H2��g��+O2��g��=2H2O��g������H=-483.6kJ/mol
�ɸ�˹���ɣ���+��× -��×
-��× �ã�N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��
�ã�N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��
�ʴ�Ϊ��-92.4kJ/mol��
��2����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%������ƽ��ʱ�����������ʵ���Ϊ
��1.5mol+6mol��×80%=6mol��
���ڷ�Ӧ��N2��g��+3H2��g��?2NH3��g�� ���ʵ������١�n
1 2
0.75mol ��1.5mol+6mol��-6mol=1.5mol
����ƽ��ʱ������ת����Ϊ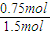 ×100%=50%��
×100%=50%��
ƽ�ⳣ��ָ������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ�����Զ��ڣ�
N2��g��+3H2��g��?2NH3��g���ķ�Ӧƽ�ⳣ��k=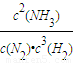 ��
��
�ٷ�ӦΪ���������С�ķ�Ӧ������ѹǿƽ�������������С�����ƶ�����������Ӧ�ƶ���ƽ�ⳣ�����䣬�ʢ���ȷ��
������Ӧ���Ũ�ȣ�ƽ��������Ӧ�ƶ���ƽ�ⳣ�����䣬�ʢ���ȷ��
��ʹ�ô������ӿ췴Ӧ���ʣ�ƽ�ⲻ�ƶ����ʢ۴���
�ܺϳɰ��Ƿ��ȷ�Ӧ�������¶ȣ�ƽ������ȷ�Ӧ�����ƶ�����������Ӧ�ƶ�����ƽ�ⳣ�����ʢܴ���
�ʴ�Ϊ��50%��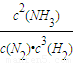 ���٢ڣ�
���٢ڣ�
��3����Ӧ���Ȼ�����ɣ���Ԫ�صĻ��ϼ۽��ͣ�����������ԭ��Ӧ����֪��Ԫ�ر�����Ϊ��������Ӧ����ʽΪ
8NH3+3Cl2=N2+6NH4Cl��
�ʴ�Ϊ��8NH3+3Cl2=N2+6NH4Cl��
��4����ϳ����ԣ��������ӵ�Ũ�ȵ���笠�����Ũ�ȣ�pH=a�İ�ˮ������һˮ�ϰ���������ʣ�����ˮ��Ũ�ȴ���
10-14+a�����ڵ������ϣ��������������ӵ�Ũ�ȴ���10-14+a����������������Ũ�ȴ���10-14+a�����������pH��14-a��
�ʴ�Ϊ��С�ڣ�
��������Ŀ�ۺ��Խϴ��漰��˹���ɡ���ѧƽ����㡢ƽ���ƶ���������ԭ��Ӧ����ҺpH�ȣ��Ѷ��еȣ�ע�⣨4����������Ũ�ȵ��жϣ�һˮ�ϰ���������ʣ���ˮ��Ũ��Զ����������Ũ�ȣ�
��2������ѹǿ֮�ȵ������ʵ���֮�ȼ����ƽ������������ʵ��������ò���������μӷ�Ӧ�ĵ��������ʵ���������ת���ʶ�����㵪����ת���ʣ�
ƽ�ⳣ��ָ������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��
ƽ�ⳣ��ֻ���¶�Ӱ�죬ƽ�ⳣ�����䣬�¶Ȳ��ܱ仯����Ϸ�Ӧ�ص㣬�������������ƽ���Ӱ���������жϣ�
��3����Ӧ���Ȼ�����ɣ���Ԫ�صĻ��ϼ۽��ͣ�����������ԭ��Ӧ����֪��Ԫ�ر�����Ϊ����������������ԭ��Ӧ��ƽд������ʽ��
��4����ϳ����ԣ��������ӵ�Ũ�ȵ���笠�����Ũ�ȣ�����һˮ�ϰ���������ʣ�����ˮ��Ũ�ȴ���10-14+a���������������ӵ�Ũ�ȴ��ڴ���10-14+a����������������Ũ�ȴ���10-14+a��
����⣺��1����֪����N2��g��+O2��g��=2NO��g������H=180.5kJ/mol
��4NH3��g��+5O2��g��=4NO��g��+6H2O��g������H=-905kJ/mol
��2H2��g��+O2��g��=2H2O��g������H=-483.6kJ/mol
�ɸ�˹���ɣ���+��×
 -��×
-��× �ã�N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��
�ã�N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol���ʴ�Ϊ��-92.4kJ/mol��
��2����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%������ƽ��ʱ�����������ʵ���Ϊ
��1.5mol+6mol��×80%=6mol��
���ڷ�Ӧ��N2��g��+3H2��g��?2NH3��g�� ���ʵ������١�n
1 2
0.75mol ��1.5mol+6mol��-6mol=1.5mol
����ƽ��ʱ������ת����Ϊ
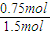 ×100%=50%��
×100%=50%��ƽ�ⳣ��ָ������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ�����Զ��ڣ�
N2��g��+3H2��g��?2NH3��g���ķ�Ӧƽ�ⳣ��k=
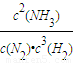 ��
���ٷ�ӦΪ���������С�ķ�Ӧ������ѹǿƽ�������������С�����ƶ�����������Ӧ�ƶ���ƽ�ⳣ�����䣬�ʢ���ȷ��
������Ӧ���Ũ�ȣ�ƽ��������Ӧ�ƶ���ƽ�ⳣ�����䣬�ʢ���ȷ��
��ʹ�ô������ӿ췴Ӧ���ʣ�ƽ�ⲻ�ƶ����ʢ۴���
�ܺϳɰ��Ƿ��ȷ�Ӧ�������¶ȣ�ƽ������ȷ�Ӧ�����ƶ�����������Ӧ�ƶ�����ƽ�ⳣ�����ʢܴ���
�ʴ�Ϊ��50%��
 ���٢ڣ�
���٢ڣ���3����Ӧ���Ȼ�����ɣ���Ԫ�صĻ��ϼ۽��ͣ�����������ԭ��Ӧ����֪��Ԫ�ر�����Ϊ��������Ӧ����ʽΪ
8NH3+3Cl2=N2+6NH4Cl��
�ʴ�Ϊ��8NH3+3Cl2=N2+6NH4Cl��
��4����ϳ����ԣ��������ӵ�Ũ�ȵ���笠�����Ũ�ȣ�pH=a�İ�ˮ������һˮ�ϰ���������ʣ�����ˮ��Ũ�ȴ���
10-14+a�����ڵ������ϣ��������������ӵ�Ũ�ȴ���10-14+a����������������Ũ�ȴ���10-14+a�����������pH��14-a��
�ʴ�Ϊ��С�ڣ�
��������Ŀ�ۺ��Խϴ��漰��˹���ɡ���ѧƽ����㡢ƽ���ƶ���������ԭ��Ӧ����ҺpH�ȣ��Ѷ��еȣ�ע�⣨4����������Ũ�ȵ��жϣ�һˮ�ϰ���������ʣ���ˮ��Ũ��Զ����������Ũ�ȣ�

��ϰ��ϵ�д�
�����Ŀ



 2NO(g) ��H
2NO(g) ��H ��������ͬ���������ʱ����Һ�����ԣ���������pH__________
��������ͬ���������ʱ����Һ�����ԣ���������pH__________ ������ڡ�����С�ڡ����ڡ�����
������ڡ�����С�ڡ����ڡ����� 2NH3��g���ش��������⣺
2NH3��g���ش��������⣺ ����c��
����c�� ����c��H2SO3��]��c��
����c��H2SO3��]��c�� ����c��NH3��H2O��
����c��NH3��H2O��