��Ŀ����
��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ����
 C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1��
C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1����д�������ʿ��ܵ�ͬ���칹��Ľṹ��ʽ��������______
��2��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��
��X�ķ���ʽΪ______�������ŵ�������______
��X������е�������ͭ���·�Ӧ����Y����ѧ����ʽΪ______

���𰸡���������1���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��ӦΪC2H4���� 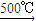 C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10�����ܵ�ͬ���칹������������춡�����֣�
C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10�����ܵ�ͬ���칹������������춡�����֣�
��2��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����������������Ϊ1-52.2%-13��%=34.8%�����л����к�C��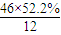 =2����H��
=2����H��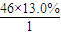 =6��
=6��
��O��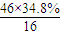 =1�������ʽΪC2H6O��Ϊ�Ҵ���
=1�������ʽΪC2H6O��Ϊ�Ҵ���
��3��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ���
����⣺��1���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��ӦΪC2H4����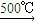 C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10��
C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10��
��Ӧ�Ľṹ��ʽ�ֱ�ΪCH3CH��CH3��CH3��CH3CH2CH2CH3���ֱ�Ϊ2-�����顢���飬
�ʴ�Ϊ��CH3CH��CH3��CH32-�����飻CH3CH2CH2CH3���飻
��2��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����������������Ϊ1-52.2%-13��%=34.8%�����л����к�C��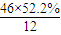 =2����H��
=2����H��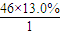 =6����O��
=6����O��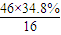 =1�������ʽΪC2H6O��Ϊ�Ҵ�
=1�������ʽΪC2H6O��Ϊ�Ҵ�
�������Ϸ�����֪X�ķ���ʽΪC2H6O���ṹ��ʽΪCH3CH2OH�����еĹ�����Ϊ�ǻ���
�ʴ�Ϊ��C2H6O�� �ǻ���
��CH3CH2OH������е�������ͭ���·�Ӧ����Y��YΪCH3CHO��
��ѧ����ʽΪ2CH3CH2OH+O2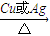 2CH3CHO+2H2O��
2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��3��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ�
�������ﺬ���Ҵ������ᣬ���߶�������ˮ�������ò��뵽Һ�����£���������������ʴ�Ϊ����ֹ��Һ������
�����ɵ��������������ڱ���̼������Һ��Ӧ�÷�Һ�ķ������룬�ʴ�Ϊ��b��
�������ӷ����������ԣ�����̼���Ʒ���2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2�����������ݣ�
�ʴ�Ϊ������ķе�ͣ�����ʱ��������������Թܼף���ʱ��������̼���ƽӴ���������Ӧ2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2�����������ݣ�
���������⿼���л�����ƶϣ�������ѧ����������������������ʵ�������Ŀ��飬ע����ճ����л���Ľṹ�����ʣ���Ŀ�Ѷ��еȣ�
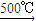 C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10�����ܵ�ͬ���칹������������춡�����֣�
C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10�����ܵ�ͬ���칹������������춡�����֣���2��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����������������Ϊ1-52.2%-13��%=34.8%�����л����к�C��
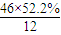 =2����H��
=2����H��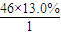 =6��
=6����O��
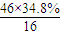 =1�������ʽΪC2H6O��Ϊ�Ҵ���
=1�������ʽΪC2H6O��Ϊ�Ҵ�����3��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ���
����⣺��1���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��ӦΪC2H4����
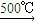 C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10��
C2H6+�ף��������ʵĻ�ѧ��������Ϊ1��1��1�����C4H10����Ӧ�Ľṹ��ʽ�ֱ�ΪCH3CH��CH3��CH3��CH3CH2CH2CH3���ֱ�Ϊ2-�����顢���飬
�ʴ�Ϊ��CH3CH��CH3��CH32-�����飻CH3CH2CH2CH3���飻
��2��ͨ����ʳ���Ϳɻ��ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����������������Ϊ1-52.2%-13��%=34.8%�����л����к�C��
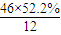 =2����H��
=2����H��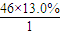 =6����O��
=6����O��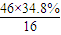 =1�������ʽΪC2H6O��Ϊ�Ҵ�
=1�������ʽΪC2H6O��Ϊ�Ҵ��������Ϸ�����֪X�ķ���ʽΪC2H6O���ṹ��ʽΪCH3CH2OH�����еĹ�����Ϊ�ǻ���
�ʴ�Ϊ��C2H6O�� �ǻ���
��CH3CH2OH������е�������ͭ���·�Ӧ����Y��YΪCH3CHO��
��ѧ����ʽΪ2CH3CH2OH+O2
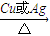 2CH3CHO+2H2O��
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
 2CH3CHO+2H2O��
2CH3CHO+2H2O�� ��3��X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����ZΪCH3COOH��WΪCH3COOCH2CH3��Ϊ�����������Ʊ�
�������ﺬ���Ҵ������ᣬ���߶�������ˮ�������ò��뵽Һ�����£���������������ʴ�Ϊ����ֹ��Һ������
�����ɵ��������������ڱ���̼������Һ��Ӧ�÷�Һ�ķ������룬�ʴ�Ϊ��b��
�������ӷ����������ԣ�����̼���Ʒ���2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2�����������ݣ�
�ʴ�Ϊ������ķе�ͣ�����ʱ��������������Թܼף���ʱ��������̼���ƽӴ���������Ӧ2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2�����������ݣ�
���������⿼���л�����ƶϣ�������ѧ����������������������ʵ�������Ŀ��飬ע����ճ����л���Ľṹ�����ʣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��
��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��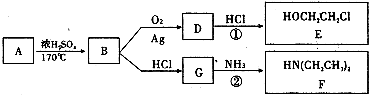

 ijͬѧ������ͼ��ʾ��ʵ��װ����ȡW��ʵ��������Թܼ����ϲ�Ϊ���ġ�������ˮ����״Һ�塣
ijͬѧ������ͼ��ʾ��ʵ��װ����ȡW��ʵ��������Թܼ����ϲ�Ϊ���ġ�������ˮ����״Һ�塣