题目内容
【题目】已知有机物A、B、C、D、E、F有以下转化关系.A的产量是衡量一个国家石油化工生产水平的标志;D能使石蕊试液变红;E是不溶于水且具有水果香味的无色液体,相对分子质量是C的2倍;F是塑料的主要成分之一,常用于制食品包装袋.结合如图关系回答问题:

(1)按要求回答下列问题:
①写出A、E的结构简式:A______、E______;
②写出B、C、D、E中官能团的名称:B______、C、______D______E、______;
③写出反应②的反应方程式:_______________________________________
(2)A与苯都是石油化工的重要产品,在一定条件下A可以转化生成苯,按要求回答下列问题:
苯可以发生取代反应,写出由苯制备溴苯的化学反应方程式:________________________
【答案】 CH2=CH2 CH3COOCH2CH3 羟基 醛基 羧基 酯基 2CH3CH2OH+O2![]() 2CH3CHO+2H2O
2CH3CHO+2H2O 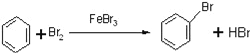
【解析】A的产量是衡量一个国家石油化工生产水平的标志,则A是乙烯,其结构简式为CH2=CH2;A和水发生加成反应生成B为CH3CH2OH,乙醇发生催化氧化生成C为CH3CHO,D能使石蕊试液变红,则D是羧酸,E是不溶于水且具有香味的无色液体,属于酯,所以B和D发生酯化反应生成E,且E的相对分子质量是C的2倍,则E的相对分子质量=44×2=88,则D的相对分子质量=88+18-46=60,D为乙酸,其结构简式为CH3COOH,E为乙酸乙酯,其结构简式为CH3COOCH2CH3,F是高分子聚合物,常用于制食品包装袋,则F是聚乙烯,其结构简式为![]() ;
;
(1)①通过以上分析知,A的结构简式为CH2=CH2、E的结构简式为CH3COOCH2CH3;
②B的结构简式为CH3CH2OH,含有羟基,C为CH3CHO,含有醛基,D的结构简式为CH3COOH,含有羧基,E的结构简式为CH3COOCH2CH3,含有官能团为酯基;
③反应②是乙醇发生氧化反应生成乙醛,反应反应方程式:2CH3CH2OH+O2![]() 2CH3CHO+2H2O;
2CH3CHO+2H2O;
(2)苯和液溴在FeBr3作催化剂条件下发生取代反应生成溴苯,由苯制备溴苯的化学反应方程式: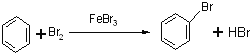 。
。

 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案

