��Ŀ����
����Ŀ��A��B��C��D��E��F��G�Ƕ�����Ԫ�أ����ڱ���B��C���ڣ�C��Eͬ���壻A��L����K���2����B�ĵ�������C�ĵ�������1����FԪ�ص�ԭ�������ڱ��а뾶��С������������D2C2��ˮ��Ӧ����C�����嵥�ʣ�����Һʹ��̪��Һ��졣G�ǵ�������ԭ�Ӱ뾶��С������Ԫ�ء�
��1��B��Ԫ�����ڱ��е�λ��_____��D������������ˮ����ĵ���ʽ____���õ���ʽ��ʾFGC���γɹ���Ϊ_______��
��2��B��FԪ�ؿ��γ�18���ӷ��ӵĵ���ʽΪ_____��
��3��A��B��C���⻯���ȶ���˳��Ϊ_______���÷���ʽ��ʾ����G�������ӵĻ�ԭ��_____�����ڻ�С�ڣ�E�������ӡ�
��4��F2C��F2E�У��е�ϸߵ���____���ѧʽ��������Ҫԭ����_____��
��5������Sn���ǹŴ����֮һ���ڵ������ڵڢ�A�����ܺ�Ũ���ᷴӦ����Sn��SO4��2�ʹ̼�����ζ���壬��Ӧ�Ļ�ѧ����ʽΪ_______��
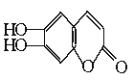
���𰸡��ڶ�������A�� ![]()
![]()
![]() CH4��NH3��H2O С�� H2O ˮ���Ӽ������� Sn+4H2SO4=Sn��SO4��2+2SO2�� +4H2O
CH4��NH3��H2O С�� H2O ˮ���Ӽ������� Sn+4H2SO4=Sn��SO4��2+2SO2�� +4H2O
��������
��A��L����K���2����֪��AΪCԪ�أ�FԪ�ص�ԭ�������ڱ��а뾶��С����FΪHԪ�أ��ɳ���������D2C2��ˮ��Ӧ����C�����嵥�ʣ�����Һʹ��̪��Һ����֪���÷�ӦΪ����������ˮ��Ӧ�����������ƺ���������DΪNaԪ�ء�CΪOԪ�أ�B�ĵ�������C�ĵ�������1������BΪNԪ�أ�C��Eͬ���壬��EΪSԪ�أ�G�ǵ�������ԭ�Ӱ뾶��С������Ԫ�أ���GΪClԪ�أ��ۺ����Ϸ�����֪AΪCԪ�ء�BΪNԪ�ء�CΪOԪ�ء�DΪNaԪ�ء�FΪHԪ�ء�GΪClԪ�ء�
��1��BΪNԪ�أ�λ�����ڱ��ڶ����ڢ�A�壻DΪNaԪ�أ�NaԪ�ص�����������ˮ����Ϊ���ӻ�����NaOH������ʽΪ![]() ��FGCΪ���ۻ�����HClO��HClO�ĽṹʽCl��O��H���õ���ʽ��ʾHClO���γɹ���Ϊ
��FGCΪ���ۻ�����HClO��HClO�ĽṹʽCl��O��H���õ���ʽ��ʾHClO���γɹ���Ϊ![]() ���ʴ�Ϊ���ڶ����ڢ�A�壻
���ʴ�Ϊ���ڶ����ڢ�A�壻![]() ��
��![]() ��
��
��2��BΪNԪ�أ�FΪHԪ�أ������γɵ�18���ӷ���Ϊ���ۻ�����N2H4������ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3���ǽ���Ԫ��C��N��O�ķǽ�����ǿ��˳��ΪC��N��O��Ԫ�طǽ�����Խǿ���⻯��Խ�ȶ������⻯���ȶ���˳��ΪCH4��NH3��H2O���ǽ�����S��Cl��Ԫ�طǽ�����Խǿ�����Ӧ���ӻ�ԭ��Խ�����������ӻ�ԭ��С�������ӣ��ʴ�Ϊ��CH4��NH3��H2O��С�ڣ�
��4��F2CΪH2O��F2EΪH2S��H2O���Ӽ��γ�������е����H2S���ʴ�Ϊ��H2O��ˮ���Ӽ���������
��5���������֪���ܺ�Ũ���ᷴӦ����Sn��SO4��2���̼�����ζ��SO2�����H2O����Ӧ�Ļ�ѧ����ʽΪSn+4H2SO4=Sn��SO4��2+2SO2�� +4H2O���ʴ�Ϊ��Sn+4H2SO4=Sn��SO4��2+2SO2�� +4H2O��

����Ŀ���������ʵķ����У�������ϵ�����ϡ�X����Y��Y����Z������
ѡ�� | X | Y | Z |
A | �����廯���� | �������������� |
|
B | ֬���廯���� | ��״���������� | CH3COOH(����) |
C | ��״������ | �����廯���� | ����ͬϵ�� |
D | �������� | ������ |
|
A. A B. B C. C D. D