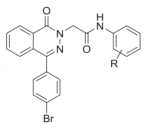
��Ŀ����
����Ŀ���ش��������⣺
��1��Ǧ���ص��ܷ�ӦΪ��Pb + PbO2 + 2H2SO4 ![]() 2PbSO4 + 2H2O���ŵ�ʱ��������ӦʽΪ___________�����ʱ��������ӦʽΪ___________��
2PbSO4 + 2H2O���ŵ�ʱ��������ӦʽΪ___________�����ʱ��������ӦʽΪ___________��
��2��������ͼװ�ã�����ģ�����ĵ绯ѧ������
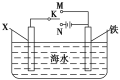
����XΪʯī��Ϊ�������ĸ�ʴ��������K���ڣδ����õ绯ѧ��������Ϊ___________��
����XΪп������K����M�����õ绯ѧ��������Ϊ__________��
��3���ҹ��ĿƼ���ԱΪ������SO2����Ⱦ������ԭ���ԭ���������ͼ2װ����SO2��O2�Ʊ����ᣬ�缫A��BΪ��IJ��ϡ�
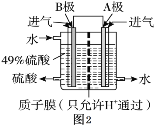
�� A���ĵ缫��Ӧʽ��________��
�� B���ĵ缫��Ӧʽ��________��
���𰸡�Pb + SO42-��2e-�� PbSO4 PbSO4 + 2H2O-2e-��PbO2 + 4H+ + SO42- ��ӵ��������������� ������������������ 4H+ + O2 + 4e-��2H2O SO2 + 2H2O - 2e- �� SO42- + 4H+
��������
(1)�ŵ�ʱ����װ����ԭ��أ�������Ǧʧ���ӷ���������Ӧ�����ʱ����װ���ǵ��أ�����ʧ���ӷ���������Ӧ��
(2)��ԭ��������������������Ľ�����������
(3)��ԭ����У�������ʧ���ӱ����������Ը�����Ͷ�ŵ������Ƕ�������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ�������Ͷ�ŵ������������������������õ��Ӻ������ӷ�Ӧ����ˮ�����������ˮ�ij��ڷ���֪��B���Ǹ�����A�����������ݴ���д�缫��Ӧʽ��
��(1)�ŵ�ʱ����װ����ԭ��أ�������Ǧʧ���ӷ���������Ӧ����Pb+SO42--2e-=PbSO4���ڳ��ʱ����װ���ǵ��أ�����������Ǧʧ���ӷ���������Ӧ����PbSO4+2H2O-2e-=PbO2+4H++SO42-���ʴ�Ϊ��Pb+SO42--2e-=PbSO4��PbSO4+2H2O-2e-=PbO2+4H++SO42-��
(2)����XΪʯī��Ϊ�������ĸ�ʴ��������K����N������װ�ù��ɵ��أ��������������������õ绯ѧ��������Ϊ��ӵ������������������ʴ�Ϊ����ӵ�����������������
����XΪп������K����M������װ�ù���ԭ��أ�п��ʧ�������������������������������õ绯ѧ��������Ϊ�����������������������ʴ�Ϊ������������������������
(3)��ԭ����У�������ʧ���ӱ����������Ը�����Ͷ�ŵ������Ƕ�������B���Ǹ�����������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ��缫��Ӧʽ��SO2+2H2O-2e-=SO42-+4H+��������Ͷ�ŵ���������������A���������������������õ��Ӻ������ӷ�Ӧ����ˮ���缫��Ӧʽ��4H++O2+4e-=2H2O���ʴ�Ϊ����4H++O2+4e-=2H2O����SO2+2H2O-2e-=SO42-+4H+��

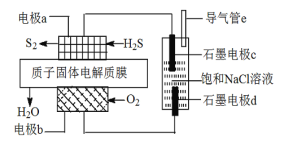
����Ŀ�����Ȼ�����S2Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������������ı��������ȷ�ճ�������Ӳ�����ʡ��������Ͽ�֪S2Cl2�����������ʣ�
��1����ȡ����S2Cl2
�������� | ���� | ɫ̬ | �ӷ��� | �۵� | �е� |
�綾 | ���ɫҺ�� | �ӷ� | -76�� | 138�� | |
��ѧ���� | ��300��������ȫ�ֽ⣻ ��S2Cl2+Cl2 �������Ȼ�������Ӵ���������ȼ�յ�Σ�գ� �����Ȼ���ˮ�ֽ���ȣ��ų���ʴ�������� | ||||
ʵ���ҿ�������������������110��140����Ӧ�Ƶ�S2Cl2��Ʒ��
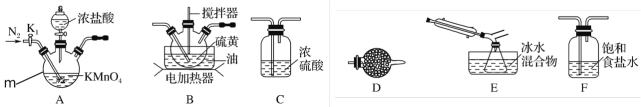
������m������Ϊ___________��װ��F���Լ���������__________��
��װ������˳��A��_______________��E��D��
��ʵ��ǰ��K1��ͨ��һ��ʱ��ĵ����ž�װ���ڿ�����ʵ�����ֹͣ���Ⱥ���ͨ��һ��ʱ��ĵ�������Ŀ����_____________��
��Ϊ�����S2Cl2�Ĵ��ȣ�ʵ��Ĺؼ��ǿ��ƺ��¶Ⱥ�_____________��
��2��S2Cl2��ˮǿ�ҷ�Ӧ�����������÷�Ӧ�Ļ�ѧ����ʽ2S2Cl2+2H2O=SO2��+3S��+4HCl����ͬѧΪ����֤������������ˮ�����ɵ���������ͨ����������ϡ����Ļ����Һ��Ʒ����Һ��NaOH��Һ���÷���_________����������������������������ԭ����___________��
��3��ijͬѧΪ�˲ⶨS2Cl2��ˮ��Ӧ�����ɵ�����X�ڻ�������е�������������������ʵ�鷽����

��W��Һ������____________�����ţ���
aH2O2��Һ bKMnO4��Һ�������ữ�� c��ˮ
�ڸû������������X���������Ϊ_____________���ú�V��m��ʽ�ӱ�ʾ����