��Ŀ����
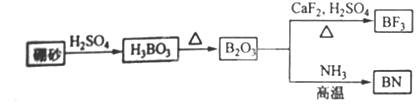
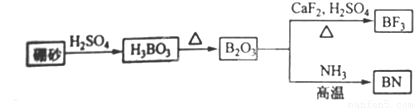
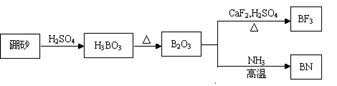
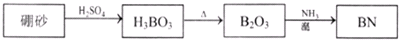
������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
��ش��������⣺
1����B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������ �� ��
2����̬Bԭ�ӵĵ����Ų�ʽΪ ��B��N��ȣ��縺�Խϴ���� ��BN��BԪ�صĻ��ϼ�Ϊ ��
3����BF3�����У�F-B-F�ļ����� ��Bԭ�ӵ��ӻ��������Ϊ ��BF3����NaF���ÿ�����NaBF4��BF4-�����幹��Ϊ ��
4������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ �����������Ϊ ��
5�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к��� ����ԭ�ӡ� ����ԭ�ӣ�������������ܶ��� g/cm3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
��15�֣�
1��B2O3+3CaF2+3H2SO4 = 2BF3��+3CaSO4+3H2O��
B2O3+2NH3 2BN+3H2O��
2BN+3H2O��
2��1s22s22p1��N��+3��
3��120�㣬sp2���������壻
4�����ۼ������Ӽ���������
5��4��4��4��25/(NA��(361.5��10-10)3)��
��������
���������1����ͼ��֪B2O3��CaF2��H2SO4��Ӧ������BF3��ͬʱ��Ӧ�ò�������ƺ�ˮ������ʽΪB2O3+3CaF2+3H2SO4 2BF3��+3CaSO4+3H2O��B2O3�백���ڸ����·�Ӧ������BN������ʽΪ
2BF3��+3CaSO4+3H2O��B2O3�백���ڸ����·�Ӧ������BN������ʽΪ
B2O3+2NH3 2BN+3H2O���ʴ�Ϊ��B2O3+3CaF2+3H2SO4
2BN+3H2O���ʴ�Ϊ��B2O3+3CaF2+3H2SO4 2BF3��+3CaSO4+3H2O��B2O3+2NH3
2BF3��+3CaSO4+3H2O��B2O3+2NH3 2BN+3H2O��
2BN+3H2O��
2����̬Bԭ�ӵĵ����Ų�ʽΪ1s22s2sp1��B��N ��λ�ڵڶ����ڣ��縺�Դ����������εݼ�������N�ĵ縺�Դ���B��BN��BԪ�صĻ��ϼ�Ϊ+3���ʴ�Ϊ��1s22s2sp1��N��+3��
3�����ݼ۲���ӶԻ������ۣ������BF3�ŶԵ��Ӷ���=(1/2)����a-xb��=(1/2)����4-4��1��=0�����Ҽ۲���Ӷ���Ϊ3������BF3����Ϊƽ���������νṹ������Ϊ120�㣬�ӻ���ʽΪsp2��BF4-����ԭ�ӵŶԵ��Ӷ���==(1/2)����a-xb��==(1/2)����4-4��1��=0����۲���Ӷ���Ϊ4��������ṹΪ�������壮�ʴ�Ϊ��120�㣻sp2���������壻
4��B��N�����ڷǽ���Ԫ�أ������γɵĻ�ѧ���Ǽ��Թ��ۼ�������ʯī�ṹ��֪�������������У������֮�俿���Ӽ���������ϣ��ʴ�Ϊ�����ۼ������Թ��ۼ��������Ӽ���������
5�����ݽ��ʯ�Ľṹ�����жϳ����ʯ��һ�������к��е�̼ԭ����=8��=(1/8)+6��=(1/2)+4=8�����һ�������������к���4��Nԭ�Ӻ�4��Bԭ�ӣ�һ�������е�����Ϊ25g/ NA��4��һ������������������ǣ�361.5cm��3�����������������ܶ���(25��4)/ [(361.5��10-101��)3NA] g?cm-3��
���㣺Ԫ�ص����ܡ��縺�Եĺ��弰Ӧ�� ԭ�Ӻ�����ӵ��ܼ��ֲ� ���ܡ����������Ǽ���Ӧ�� �����ļ���
���������⿼���Ϊȫ�棬�漰����ѧ����ʽ����д�������Ų�ʽ�����ӿռ乹�͡��ӻ����͵��ж��Լ��йؾ���ļ��㣬��������н�ǿ�ķ����Ժ����ԣ�ѧϰ��ע���ܽ������д�����Ų�ʽ������жϷ��ӿռ乹���Լ��йؾ������ȷ�����

 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
 ����B3N3H6�Ķ��ȴ�����
����B3N3H6�Ķ��ȴ�����