��Ŀ����
�������ӷ���ʽ��ȷ���ǣ� ��
| A��Ca��HCO3��2��Һ�еμ�����NaOH��Һ Ca2++HCO3�C+OH�C = CaCO3��+ H2O |
| B��NaHSO4��Һ�еμ�����Ba��OH��2��Һ H++SO42-+Ba2++OH�C= BaS04��+H2O |
| C��FeBr2��Һ��ͨ�˹���C12 2Fe2++6Br �C+4C12 = Fe3++3Br2+8C1�� |
| D��FeSO4��Һ�м���H2O2��Һ�ķ�Ӧ: Fe2++2H2O2+4H+=Fe3++4H2O |
A
�������������Aѡ���ȷ��Bѡ�ӦΪ2H++SO42-+Ba2++2OH�C= BaS04��+2H2O����B����Cѡ�����Ԫ�ز��غ㣬��ɲ��غ㡣Dѡ���ɲ��غ㡣
���㣺���ӷ���ʽ��д��

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
�������ӷ���ʽ��д��ȷ����:
| A��̼�������Һ�м��������NaOH��Һ�� Ca2����HCO3�D��OH�D=CaCO3����H2O |
| B��NaHSO4��Һ��Ba(OH)2��Һ��Ϻ������ԣ�Ba2����OH����H����SO42����BaSO4����H2O |
| C��FeI2��Һ��ͨ������Cl2��2 Fe2�� �� Cl2="2" Fe3�� �� 2Cl�� |
| D����NH4Al��SO4��2��Һ�е���Ba��OH��2��ҺǡʹSO42-������ȫNH4++Al3++2Ba2++2SO42-+4OH- =Al(OH)3��+NH3��H2O+2BaSO4�� |
���и�ʽ�У�������ȷ�ĵ��뷽��ʽ����
| A��HCO3- = CO32- + H+ | B��HCO3- ��OH- = H2O + CO32- |
| C��NH3+ H+ = NH4+ | D��NH3·H2O 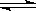 NH4+ + OH- NH4+ + OH- |
�������ֱ����뷴Ӧ����ʽ��Ӧ����ȷ����
| A���������е���AgNO3��Һ�������е���Ԫ�أ�Ag++Br��=AgBr�� |
| B������̼������Һϴ�����ۣ�CO32��+H2O=HCO3��+OH�� |
C���ö��Ե缫��ⱥ��KCl��Һ��2H++2Cl�� H2��+Cl2�� H2��+Cl2�� |
| D��FeSO4��Һ��˫��ˮ��ϡ�����ϣ�2Fe2++H2O2+2H+=2Fe3++2H2O |
���н�����ʵ�����ӷ���ʽ��ȷ����( )
| A����������Һ�м�������������Һ�������������Al3++2SO42-+2Ba2++4OH-=2BaSO4��+AlO2-+2H2O |
| B����Ca(HCO3)2��Һ�м������NaOH��Һ���а�ɫ�������ɣ�Ca2++HCO3-+OH- =CaCO3��+H2O |
C���ö��Ե缫���MgCl2��Һ��2Cl-+2H2O 2OH- +Cl2��+ H2�� 2OH- +Cl2��+ H2�� |
| D��NaClO��Һ��ͨ��������SO2��ClO-+H2O+SO2=Cl-+SO42-+2H+ |
��ij������Һ�����ܺ���Br�D��SO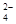 ��H2SO3��NH
��H2SO3��NH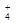 ���ֱ��������ʵ�飺
���ֱ��������ʵ�飺
�ټ���ʱ�ų����������ʹƷ����Һ��ɫ
�ڼӼ�������Ժ���ʱ�ų����������ʹ��ʪ�ĺ�ɫʯ����ֽ������
�ۼ�����ˮʱ����Һ���Ի�ɫ���ټ���BaCl2��Һʱ�������İ�ɫ����������ϡ����
�����������ʲ���ȷ��������Һ���Ƿ���ڵ��� �� ��
| A��Br�D | B��SO42- | C��H2SO3 | D��NH4+ |
������������ǿ��������ܵ������
���Ȼ�����Һ ���Ȼ�粒��� ��ͭ ��ʯī ������NaOH ��ϡ���� ������
| A���� | B���٢ڢ� |
| C���ڢݢޢ� | D���٢ۢܢݢ� |
�������ӷ���ʽ����ȷ����
| A���Ҵ��ͽ����Ʒ�Ӧ����CH3CH2OH+��Na ����CH3CH2O��+ Na++H2�� |
B����������NaOH����Һ�м��ȣ�CH3CH2Cl + OH��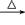 CH3CH2OH+Cl�� CH3CH2OH+Cl�� |
| C��������NaOH��Һ�кͷ�Ӧ��H+ + OH�� = H2O |
D����������Һ��ͨ������CO2�� |
������H+ + OH- = H2O���ӷ���ʽ��ʾ�Ļ�ѧ��Ӧ��
| A�����������������Һ�ķ�Ӧ | B�������������ͭ��Һ�ķ�Ӧ |
| C������ͳ���ʯ��ˮ�ķ�Ӧ | D�����������������Һ��Ӧ |