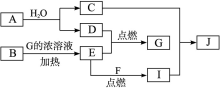
��Ŀ����
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+�� ��
��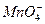 ��
��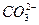 ��
��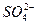 �е�������������ɣ�ȡ��Һ������������ʵ�飺
�е�������������ɣ�ȡ��Һ������������ʵ�飺

(1)����A�ijɷ���______________������B�ijɷ���_______________��(�ѧʽ)
(2)X��Һ��һ�����ڵ�������_______________��
(3)д��������з�����Ӧ�����е����ӷ���ʽ_______________��
(4)д����������γɰ�ɫ���������ӷ���ʽ_______________��
(5)ͨ������ʵ�飬��ɫ�����ҵ���ɿ�����_______________��ֻҪ���һ���ĺ���ʵ��Ϳ���ȷ���ó�������ɣ��÷�����______________________________��
 ��
��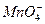 ��
��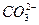 ��
��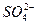 �е�������������ɣ�ȡ��Һ������������ʵ�飺
�е�������������ɣ�ȡ��Һ������������ʵ�飺
(1)����A�ijɷ���______________������B�ijɷ���_______________��(�ѧʽ)
(2)X��Һ��һ�����ڵ�������_______________��
(3)д��������з�����Ӧ�����е����ӷ���ʽ_______________��
(4)д����������γɰ�ɫ���������ӷ���ʽ_______________��
(5)ͨ������ʵ�飬��ɫ�����ҵ���ɿ�����_______________��ֻҪ���һ���ĺ���ʵ��Ϳ���ȷ���ó�������ɣ��÷�����______________________________��
(1)CO2NH3
(2)Na+�� ��
��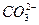
(3)C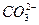 +2H+====CO2��+H2O
+2H+====CO2��+H2O +4H+====Al3++2H2O
+4H+====Al3++2H2O
(4)Al3++3 ====Al(OH)3��+3CO2��
====Al(OH)3��+3CO2��
(5)BaCO3��BaCO3��BaSO4�Ļ�����ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ����
(2)Na+��
 ��
��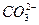
(3)C
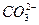 +2H+====CO2��+H2O
+2H+====CO2��+H2O +4H+====Al3++2H2O
+4H+====Al3++2H2O(4)Al3++3
 ====Al(OH)3��+3CO2��
====Al(OH)3��+3CO2��(5)BaCO3��BaCO3��BaSO4�Ļ�����ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ����
����Һ����ɫ���ų���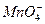 �ĸ��ţ��ܺ����ᷴӦ�����������
�ĸ��ţ��ܺ����ᷴӦ�����������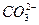 ��
��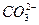 �Ĵ����ų���Ag+��Ba2+��Al3+������ԭ��Һ�к��е�������ֻ����Na+����̼�������Һ��Ӧ���ɰ�ɫ����������ֻ����Al3+(��ԭ��Һ�е�
�Ĵ����ų���Ag+��Ba2+��Al3+������ԭ��Һ�к��е�������ֻ����Na+����̼�������Һ��Ӧ���ɰ�ɫ����������ֻ����Al3+(��ԭ��Һ�е� ����������ᷴӦ����)������ԭ��Һ�к���
����������ᷴӦ����)������ԭ��Һ�к��� ��������ֻ��ΪAl(OH)3����������ٵķ�ӦΪ��
��������ֻ��ΪAl(OH)3����������ٵķ�ӦΪ��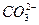 +2H+====CO2��+H2O��
+2H+====CO2��+H2O�� +4H+====Al3++2H2O�������ʱ�γɰ�ɫ���������ӷ���ʽΪAl3++3
+4H+====Al3++2H2O�������ʱ�γɰ�ɫ���������ӷ���ʽΪAl3++3 ====Al(OH)3��+3CO2����������Ba(OH)2�ṩ�˴�����OH-��Ba2+����������BΪNH3����ɫ�����ҿ�����BaCO3��BaCO3��BaSO4�Ļ�������ȷ�������鷽��Ϊ�ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ���
====Al(OH)3��+3CO2����������Ba(OH)2�ṩ�˴�����OH-��Ba2+����������BΪNH3����ɫ�����ҿ�����BaCO3��BaCO3��BaSO4�Ļ�������ȷ�������鷽��Ϊ�ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ���
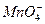 �ĸ��ţ��ܺ����ᷴӦ�����������
�ĸ��ţ��ܺ����ᷴӦ�����������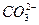 ��
��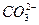 �Ĵ����ų���Ag+��Ba2+��Al3+������ԭ��Һ�к��е�������ֻ����Na+����̼�������Һ��Ӧ���ɰ�ɫ����������ֻ����Al3+(��ԭ��Һ�е�
�Ĵ����ų���Ag+��Ba2+��Al3+������ԭ��Һ�к��е�������ֻ����Na+����̼�������Һ��Ӧ���ɰ�ɫ����������ֻ����Al3+(��ԭ��Һ�е� ����������ᷴӦ����)������ԭ��Һ�к���
����������ᷴӦ����)������ԭ��Һ�к��� ��������ֻ��ΪAl(OH)3����������ٵķ�ӦΪ��
��������ֻ��ΪAl(OH)3����������ٵķ�ӦΪ��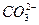 +2H+====CO2��+H2O��
+2H+====CO2��+H2O�� +4H+====Al3++2H2O�������ʱ�γɰ�ɫ���������ӷ���ʽΪAl3++3
+4H+====Al3++2H2O�������ʱ�γɰ�ɫ���������ӷ���ʽΪAl3++3 ====Al(OH)3��+3CO2����������Ba(OH)2�ṩ�˴�����OH-��Ba2+����������BΪNH3����ɫ�����ҿ�����BaCO3��BaCO3��BaSO4�Ļ�������ȷ�������鷽��Ϊ�ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ���
====Al(OH)3��+3CO2����������Ba(OH)2�ṩ�˴�����OH-��Ba2+����������BΪNH3����ɫ�����ҿ�����BaCO3��BaCO3��BaSO4�Ļ�������ȷ�������鷽��Ϊ�ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ���
��ϰ��ϵ�д�
�����Ŀ
 ����������������
���������������� �Լ� �� �����ӷ���ʽ
�Լ� �� �����ӷ���ʽ 
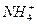 ��Al3+����Һ�У������������������ˮ��Һ�����Ƚ��裬�ټ���������ᣬ��Һ�м��ٵ���������
��Al3+����Һ�У������������������ˮ��Һ�����Ƚ��裬�ټ���������ᣬ��Һ�м��ٵ���������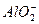 +2H2O
+2H2O ====CaCO3����
====CaCO3����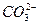 ��2H2O
��2H2O