��Ŀ����
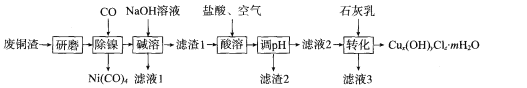
����Ŀ����������(Na2FeO4)���к�ǿ��������,��һ�����͵���ɫ��ˮ����������ҵ����������(��Ҫ�ɷ���FeCO3������SiO2)Ϊԭ���Ʊ��������������������£�

(1)Na2FeO4����Ԫ�صĻ��ϼ�Ϊ___,������������ɱ������ʱ�Ļ�ѧ��Ӧ����Ϊ____(����������ԭ��Ӧ���������ֽⷴӦ���������Ϸ�Ӧ��)��
(2)������������,������м��ʱ�ܷ��ýϱ��˵�Ca(OH)2���NaOH_____(������������������),������___________��
(3)�������28%��ϡ������Ҫ��98%��Ũ��������,����ʱ���貣����������Ͳ��,��____(����ĸ���)��������м���Fe2+ȫ��ת����Fe3+�ķ����ǣ�__________��
A������ƿ B���ձ� C����ƿ D�������� E����ʽ�ζ���;
(4)������г�����Na2FeO4��,����NaCl����,�����ӷ���ʽΪ_________����֪���������Na2FeO4��Һ�м��������������ƹ���õ�����Һ,�����a������Ϊ_____��
(5)������,ÿ���0.5mol��FeO42-���� NaClO��������Ϊ_____��
���𰸡�+6 ������ԭ��Ӧ ���� CaSiO3 ������ˮ���������SiO2������������Ҳ�ɣ� BD ȡ����������Һ���Թܣ���������K3Fe(CN)6��Һ��������ɫ��������Fe2�� �Ѿ�ȫ��ת����Fe3�� 2Fe3����3ClO����10OH��=2FeO42����3Cl����5H2O ���� 74.5g
��������
��ҵ����������Ҫ�ɷ���FeCO3������SiO2������NaOH�ܽ�SiO2��ȥ�����������28%��ϡ�����ܽ������������ӣ���NaClO���������������������ӣ���Ȼ�����NaOH��NaClO������Ӧ��2Fe3��+3ClO��+10OH��=2FeO42��+3Cl��+5H2O���õ��������ƣ�Na2FeO4����Һ�������ṹ����Ũ������ȴ�ᾧ�õ���Ʒ�������ơ�
��1��Na2FeO4�л��ϼ۴�����Ϊ�㣻
��2��CaSiO3 ������ˮ��
��3����������һ����������Һ�IJ��裺���㡢�������ܽ⣬ȷ�����õ�������
����K3Fe(CN)6��Һ������Fe2+��
��4��������г�����Na2FeO4�⣬����NaCl���ɣ����������غ�д�����ӷ���ʽ��
���������Һ��Ӧ�ù��ˣ�
��5�������ϣ��ɵ����غ㣬����+2�۱��+6�ۣ�����ϵʽFeO42-��2NaClO���㡣
��1��Na2FeO4�л��ϼ۴�����Ϊ�㣬��FeԪ�صĻ��ϼ�Ϊ+6�ۣ�
�������ƾ��к�ǿ�������ԣ�������������ɱ������ʱ�Ļ�ѧ��Ӧ����Ϊ������ԭ��Ӧ��
��2����NaOH�ܽ�SiO2���ɹ��������ܽ⣬�Ӷ���FeCO3�������ȥ�������ýϱ��˵�Ca(OH)2���NaOH��CaSiO3 ������ˮ���������SiO2��
��3����������һ����������Һ�IJ��裺���㡢�������ܽ⣬ȷ�����õ���������ͷ�ιܡ���Ͳ�����������ձ�����ѡ��BD��
������м���Fe2+ȫ��ת����Fe3+�ķ����ǣ�ȡ����������Һ���Թܣ���������K3Fe(CN)6��Һ��������ɫ��������Fe2�� �Ѿ�ȫ��ת����Fe3����
��4��������г�����Na2FeO4�⣬����NaCl����,�����ӷ���ʽΪ2Fe3����3ClO����10OH��=2FeO42����3Cl����5H2O��
��֪���������Na2FeO4��Һ�м��������������ƹ���õ�����Һ�����������Һ��Ӧ�ù��ˣ������a������Ϊ���ˡ�
��5�������ϣ��ɵ����غ㣬����+2�۱��+6�ۣ�����ϵʽFeO42-��2NaClO���㣬ÿ���0.5mol��FeO42-���� NaClO��������Ϊ74.5g��