��Ŀ����
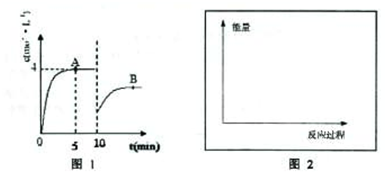
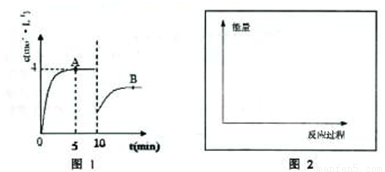
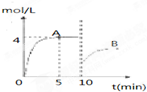 ��һ�������£��ϳ����е�������������ʼŨ�ȷֱ�Ϊa mol?L-1��b mol?L-1����ӦΪ��N2+3H2?2NH3��������Ũ����ʱ��仯��ͼ��ʾ��
��һ�������£��ϳ����е�������������ʼŨ�ȷֱ�Ϊa mol?L-1��b mol?L-1����ӦΪ��N2+3H2?2NH3��������Ũ����ʱ��仯��ͼ��ʾ����1����Ӧ��5minʱ��������Ӧ����
1.2mol/��L?min��
1.2mol/��L?min��
��A��ƽ�ⳣ��Ϊ| 16 |
| (a-2)��(b-4)3 |
| 16 |
| (a-2)��(b-4)3 |
��2����10minʱ��ȡ�Ĵ�ʩ��
�������ɵİ��������Ͱ�����Ũ��
�������ɵİ��������Ͱ�����Ũ��
����3��-50��Cʱ��Һ���������µ��룺2NH3?NH4++NH-2��K=2��10-12����Һ���м���NH4Cl���壬K
=
=
2��10-12�����������������=������4����֪2A2��g��+B2��g���T2C3��g������H=-a kJ/mol��a��0������һ���д����Ĺ̶��ݻ��������м���2mol A2��1mol B2����500��ʱ��ַ�Ӧ��ƽ���C3��Ũ��Ϊw mol/L���ų�����b kJ������ԭ���������У�ֻ����2mol C3��500��ʱ��ַ�Ӧ��ƽ�����������c kJ��a��b��c֮��������ֹ�ϵ
b+c=a
b+c=a
���ô���ʽ��ʾ����������1����ͼ��֪����Ӧ��5minʱ��ƽ��״̬��������Ũ��Ϊ4mol/L������v=
����v��NH3��������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��H2����
��������ʽ����ƽ��ʱ����ֵ�Ũ�ȣ�����ƽ�ⳣ��k=
���㣻
��2����ͼ��֪��10min˲��ı�������NH3��Ũ��Ϊԭƽ���һ�룬�������Ũ�����ı�����ƽ�������ɰ����ķ����ƶ���Ӧ�������߰��������ʵ��������Ͱ�����Ũ�ȣ�
��3������ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬����ƽ�ⳣ�����䣬����Ũ���أ�
��4����Ӧǰ���������������仯�����º����£�����2mol C3������ѧ������ת������ߣ��ɵõ�2molA2��g����1molB2��g�����뿪ʼ����2mol A2��1mol B2Ϊ��Чƽ�⣬ƽ��ʱ��Ӧ��ֵ����ʵ�����ȣ���ƽ��ʱ������C3�����ʵ���Ϊxmol�����ݷ���ʽ��������״̬����ƽ��ʱ�μӷ�Ӧ��A2��C3���ʵ������ڸ����Ȼ�ѧ����ʽ�����������ݴ˼�����
| ��c |
| ��t |
��������ʽ����ƽ��ʱ����ֵ�Ũ�ȣ�����ƽ�ⳣ��k=
| c2(NH3) |
| c(N2)?c3(H2) |
��2����ͼ��֪��10min˲��ı�������NH3��Ũ��Ϊԭƽ���һ�룬�������Ũ�����ı�����ƽ�������ɰ����ķ����ƶ���Ӧ�������߰��������ʵ��������Ͱ�����Ũ�ȣ�
��3������ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬����ƽ�ⳣ�����䣬����Ũ���أ�
��4����Ӧǰ���������������仯�����º����£�����2mol C3������ѧ������ת������ߣ��ɵõ�2molA2��g����1molB2��g�����뿪ʼ����2mol A2��1mol B2Ϊ��Чƽ�⣬ƽ��ʱ��Ӧ��ֵ����ʵ�����ȣ���ƽ��ʱ������C3�����ʵ���Ϊxmol�����ݷ���ʽ��������״̬����ƽ��ʱ�μӷ�Ӧ��A2��C3���ʵ������ڸ����Ȼ�ѧ����ʽ�����������ݴ˼�����
����⣺��1����ͼ��֪����Ӧ��5minʱ��ƽ��״̬��������Ũ��Ϊ4mol/L����v��NH3��=
=0.8mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ�
��v��H2��=
v��NH3��=
��0.8mol/��L?min��=1.2mol/��L?min����
N2+3H2?2NH3��
��ʼ��mol/L����a b 0
�仯��mol/L����2 6 4
ƽ�⣨mol/L����a-2 b-6 4
��ƽ�ⳣ��k=
=
=
�ʴ�Ϊ��1.2mol/��L?min����
��
��2����ͼ��֪��10min˲��ı�������NH3��Ũ��Ϊԭƽ���һ�룬�������Ũ�����ı�����ƽ�������ɰ����ķ����ƶ���Ӧ�������߰��������ʵ��������Ͱ�����Ũ�ȣ�
�ʴ�Ϊ���������ɵİ��������Ͱ�����Ũ�ȣ�
��3������ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬����ƽ�ⳣ�����䣬����Ũ���أ��ʴ�Ϊ��=��
��4����Ӧǰ���������������仯�����º����£�����2mol C3������ѧ������ת������ߣ��ɵõ�2molA2��g����1molB2��g�����뿪ʼ����2mol A2��1mol B2Ϊ��Чƽ�⣬ƽ��ʱ��Ӧ��ֵ����ʵ�����ȣ���ƽ��ʱ������C3�����ʵ���Ϊxmol����
���ڿ�ʼ����2mol A2��1mol B2������2A2��g��+B2��g���T2C3��g����֪���μӷ�Ӧ��A2�����ʵ���Ϊxmol���ʷų�������ΪakJ��
=0.5axkJ����b=0.5ax��
����ֻ����2mol C3���ɷ�Ӧ2C3��g��?2A2��g��+B2��g�����μӷ�Ӧ��C3�����ʵ���Ϊ��2-x��mol���ʸ÷�Ӧ���յ�����ΪakJ��
=��1-0.5x��akJ����c=��1-0.5x��a��
��b+c=a��
�ʴ�Ϊ��b+c=a��
| 4mol/L |
| 5min |
��v��H2��=
| 3 |
| 2 |
| 3 |
| 2 |
N2+3H2?2NH3��
��ʼ��mol/L����a b 0
�仯��mol/L����2 6 4
ƽ�⣨mol/L����a-2 b-6 4
��ƽ�ⳣ��k=
| c2(NH3) |
| c(N2)?c3(H2) |
| 42 |
| (a-2)��(b-4)3 |
| 16 |
| (a-2)��(b-4)3 |
�ʴ�Ϊ��1.2mol/��L?min����
| 16 |
| (a-2)��(b-4)3 |
��2����ͼ��֪��10min˲��ı�������NH3��Ũ��Ϊԭƽ���һ�룬�������Ũ�����ı�����ƽ�������ɰ����ķ����ƶ���Ӧ�������߰��������ʵ��������Ͱ�����Ũ�ȣ�
�ʴ�Ϊ���������ɵİ��������Ͱ�����Ũ�ȣ�
��3������ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬����ƽ�ⳣ�����䣬����Ũ���أ��ʴ�Ϊ��=��
��4����Ӧǰ���������������仯�����º����£�����2mol C3������ѧ������ת������ߣ��ɵõ�2molA2��g����1molB2��g�����뿪ʼ����2mol A2��1mol B2Ϊ��Чƽ�⣬ƽ��ʱ��Ӧ��ֵ����ʵ�����ȣ���ƽ��ʱ������C3�����ʵ���Ϊxmol����
���ڿ�ʼ����2mol A2��1mol B2������2A2��g��+B2��g���T2C3��g����֪���μӷ�Ӧ��A2�����ʵ���Ϊxmol���ʷų�������ΪakJ��
| xmol |
| 2mol |
����ֻ����2mol C3���ɷ�Ӧ2C3��g��?2A2��g��+B2��g�����μӷ�Ӧ��C3�����ʵ���Ϊ��2-x��mol���ʸ÷�Ӧ���յ�����ΪakJ��
| (2-x)mol |
| 2mol |
��b+c=a��
�ʴ�Ϊ��b+c=a��
���������⿼�黯ѧ��Ӧ���ʼ��㡢ƽ�ⳣ���йؼ�����Ӱ�����ء���ѧƽ��ͼ����Ӱ�����ء���Чƽ�⡢��Ӧ�ȵ��йؼ���ȣ���Ŀ�ۺ��Խϴ��Ѷ��еȣ�ע�⣨4���ж���ͬһ���淴Ӧ������ͬ�¶��£����淴Ӧ�ķ�Ӧ����ֵ��ȣ������෴��

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д�
�����Ŀ