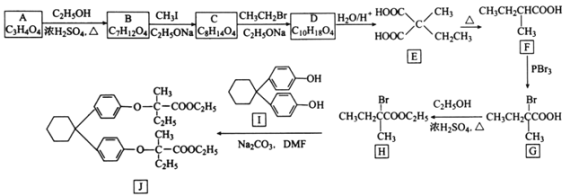
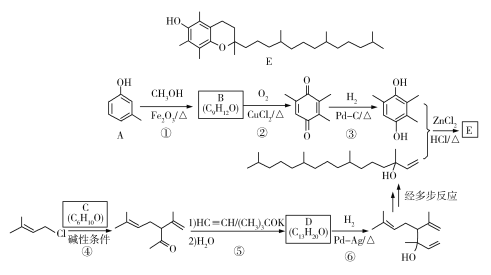
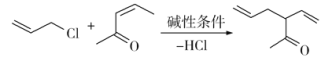
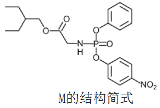
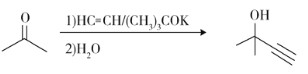��Ŀ����
����Ŀ����1����֪�������ԭ��������56 ����1����ԭ�ӵ�������_______g������NA��ʾ��
��2���ڱ�״���£�1.7g������ռ�����Ϊ_______L�������״����_____L���⺬����ͬ��Ŀ����ԭ�ӡ�
��3����֪CO��CO2�Ļ������������16.0g����״�������Ϊ8.96L�������֪��ȡ��������к�CO____g������CO2�ڱ긤״���µ����Ϊ__________L��
��4��ͬ��ͬѹ��ͬ�����H2��A����������ֱ���0.2g��l.6g��������A��ĦС����Ϊ________������A�ķ��Ӹ���Ϊ________������NA��ʾ��
��5����״���µ�aLHCl(g)����1000gˮ�У��õ��������ܶ�Ϊbg��cm-3�������������ʵ���Ũ����_____mol��L-1
���𰸡�![]() 2.24 3.36 2.8 6.72 16g/mol 0.1NA
2.24 3.36 2.8 6.72 16g/mol 0.1NA ![]()
��������
���⿼�鿼�������ʵ�����Ħ�����������ӵĸ���������Ħ����������ʵ���Ũ�ȹ�ʽ֮��Ļ��㣬�Լ�����٤�����ɵ�Ӧ�á�
��1���������ԭ��������56��������Ħ������Ϊ56g.mol-1��1molFe������Ϊ56g, 1molFeԭ�ӵĸ���ΪNA��������1������ԭ�ӵ�������![]() g��
g��
��2���ڱ�״���£�1.7g���������ʵ���Ϊ��![]() =0.1mol��V(NH3)= 0.1mol
=0.1mol��V(NH3)= 0.1mol![]() 22.4L.mol-1=2.24L����������ԭ�ӵ����ʵ���Ϊ��0.1
22.4L.mol-1=2.24L����������ԭ�ӵ����ʵ���Ϊ��0.1![]() 3=0.3mol����Ϊ�����к�����ԭ�ӵĸ��������⺬�е���ԭ�Ӹ�����ͬ��������n(H2S)
3=0.3mol����Ϊ�����к�����ԭ�ӵĸ��������⺬�е���ԭ�Ӹ�����ͬ��������n(H2S)![]() 2=0.3mol��n(H2S) =0.15mol��V(H2S)=0.15 mol
2=0.3mol��n(H2S) =0.15mol��V(H2S)=0.15 mol![]() 22.4L.mol-1=3.36L��
22.4L.mol-1=3.36L��
��3���ɷ������n(CO)+ n(CO2)= ![]() =0.4mol����28n(CO)+44n(CO2)=16.0g���ɵã�n(CO)=0.1mol��n(CO2)=0.3mol������m(CO)=0.1mol
=0.4mol����28n(CO)+44n(CO2)=16.0g���ɵã�n(CO)=0.1mol��n(CO2)=0.3mol������m(CO)=0.1mol![]() 28g.mol-1=2.8g��V(CO2)= 0.3mol
28g.mol-1=2.8g��V(CO2)= 0.3mol![]() 22.4L.mol-1=6.72L��
22.4L.mol-1=6.72L��
��4��ͬ��ͬѹ��ͬ�������H2��A��������ʵ�����ͬ��n(H2)= ![]() =0.1 mol��M(A)=
=0.1 mol��M(A)= ![]() =16g/mol��N(A)= 0.1NA
=16g/mol��N(A)= 0.1NA
��5����״���µ�aLHCl(g)�����ʵ���Ϊ![]() =
=![]() ����Һ������Ϊ
����Һ������Ϊ![]()
![]() 36.5 g/mol+1000g=(1000+
36.5 g/mol+1000g=(1000+![]() )g����Һ�����
)g����Һ�����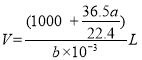 �������������ʵ���Ũ���ǣ�
�������������ʵ���Ũ���ǣ� =
=![]() mol��L-1��
mol��L-1��

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д�