��Ŀ����
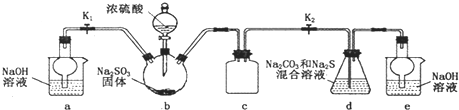
ij��ѧ��ȤС����ֻ������������ͭ�Ĺ�ҵ������ȡ�������Ȼ�����Һ���̷�����[FeSO4?7H2O]�͵������壬��̽����ҵ���ϵ������ã���ʵ�鷽�����£�
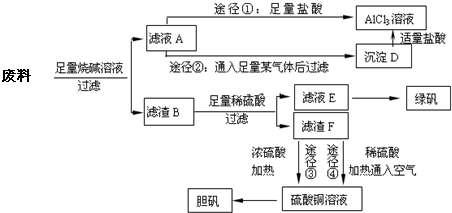
�ش��������⣺
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ______��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�;������ͨ���ij�������̬�������˹����꣬���������______���ѧʽ������ҺA�������ij�������ɳ���D�����ӷ���ʽΪ______������Ϊ�Ϻ�����;����______����ٻ�ڣ���������______��
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���Fe2+��H+�⣬������ܴ��ڵ���������______�������ӷ��ű�ʾ�����������ӵķ�����______��
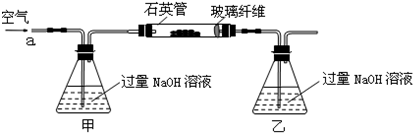
��4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ���______��______��
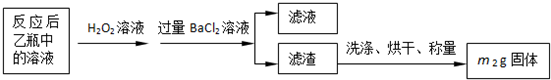
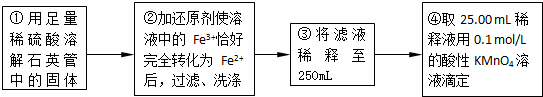
��5��;���۷����ķ�Ӧ�У�������Ũ����______�Ժ�______�ԣ�ͨ��;������ȡ������������е�ʵ��������裺�����ᡢ����ͨ���������ˡ�����Ũ������ȴ�ᾧ��______����Ȼ�������;���ܷ����ķ�ӦΪ______����һ�����ӷ���ʽ��ʾ����
�⣺��1��������Ӧ����ƫ�����ƺ����������Ի�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��2��ij�������̬�������˹����꣬������Ӧ��Ϊ������̼��������̼�Ĺ�̬Ϊ�ɱ����ɱ��������ʱ������������������ˮ������������ʵ���˹����꣬AlCl3��Һ��ͨ�������̼��̼������Ա���������ǿ�������ܷ�Ӧ����ͨ�����Ķ�����̼����̼��������ӣ�
�ʴ�Ϊ��CO2��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��;���ڣ�;���ٻ��������ᣬ�Ȼ������ʣ�
��3����ҺE�ijɷ��е����������еĶ����������ױ������е��������������������ӣ��������������ӵķ�������KSCN��Һ��Fe3++3SCN-?Fe��SCN��3Ѫ��ɫ��˵��E�к���Fe3+��
�ʴ�Ϊ��Fe3+��ȡ��ҺE�������Թ��У�����2��3�� KSCN��Һ��������Ѫ��ɫ����֤����ҺE�к���Fe3+��
��4��;���۵ķ�Ӧ��Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
;���ܵķ�Ӧ��2Cu+O2+2H2SO4��ϡ�� 2CuSO4+2H2O�ӷ�Ӧ����ʽ����������������;�������������٣�;���ܲ��������Ⱦ���������壬
2CuSO4+2H2O�ӷ�Ӧ����ʽ����������������;�������������٣�;���ܲ��������Ⱦ���������壬
�ʴ�Ϊ��������������;�������������٣�;���ܲ��������Ⱦ���������壻
��5��;���۵ķ�Ӧ��Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O���ӷ�Ӧ����ʽ���������������+6�۱�Ϊ�����е�+4��������ǿ�����ԣ��������ᷴӦ�������Σ���������������ԣ�;���ܵķ�Ӧ��2Cu+O2+2H2SO4��ϡ��
CuSO4+SO2��+2H2O���ӷ�Ӧ����ʽ���������������+6�۱�Ϊ�����е�+4��������ǿ�����ԣ��������ᷴӦ�������Σ���������������ԣ�;���ܵķ�Ӧ��2Cu+O2+2H2SO4��ϡ�� 2CuSO4+2H2O�ij����ӷ�ӦΪ2Cu+O2+4H+=2Cu2++2H2O��
2CuSO4+2H2O�ij����ӷ�ӦΪ2Cu+O2+4H+=2Cu2++2H2O��
�ʴ�Ϊ��ǿ����������ˣ� 2Cu+O2+4H+=2Cu2++2H2O��
��������1����������Ӧ����ƫ�����ƺ�������
��2���������ʵ����ʽ�Ͽ�ͼת����ϵ�����ò�Ʒ���н��
��3���������ױ������е��������������������ӣ�����������������KSCN��Һ��
��4���ӻ�����ԭ�ϵ������ʽǶȽ��
��5��ͭ��Ũ���ᷴӦ������Ũ�����ǿ�����Ժ����ԣ�ͭ���������ᷴӦ����ͭ���ӣ�
������������Ҫ�����������ӹ�ҵ��������ȡ�����ע��ӿ�ͼת�����ҳ�������Ϣ�������йصĻ�ѧ��Ӧ���н���Ѷ��еȣ�
�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��2��ij�������̬�������˹����꣬������Ӧ��Ϊ������̼��������̼�Ĺ�̬Ϊ�ɱ����ɱ��������ʱ������������������ˮ������������ʵ���˹����꣬AlCl3��Һ��ͨ�������̼��̼������Ա���������ǿ�������ܷ�Ӧ����ͨ�����Ķ�����̼����̼��������ӣ�
�ʴ�Ϊ��CO2��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��;���ڣ�;���ٻ��������ᣬ�Ȼ������ʣ�
��3����ҺE�ijɷ��е����������еĶ����������ױ������е��������������������ӣ��������������ӵķ�������KSCN��Һ��Fe3++3SCN-?Fe��SCN��3Ѫ��ɫ��˵��E�к���Fe3+��
�ʴ�Ϊ��Fe3+��ȡ��ҺE�������Թ��У�����2��3�� KSCN��Һ��������Ѫ��ɫ����֤����ҺE�к���Fe3+��
��4��;���۵ķ�Ӧ��Cu+2H2SO4��Ũ��
 CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O;���ܵķ�Ӧ��2Cu+O2+2H2SO4��ϡ��
 2CuSO4+2H2O�ӷ�Ӧ����ʽ����������������;�������������٣�;���ܲ��������Ⱦ���������壬
2CuSO4+2H2O�ӷ�Ӧ����ʽ����������������;�������������٣�;���ܲ��������Ⱦ���������壬�ʴ�Ϊ��������������;�������������٣�;���ܲ��������Ⱦ���������壻
��5��;���۵ķ�Ӧ��Cu+2H2SO4��Ũ��
 CuSO4+SO2��+2H2O���ӷ�Ӧ����ʽ���������������+6�۱�Ϊ�����е�+4��������ǿ�����ԣ��������ᷴӦ�������Σ���������������ԣ�;���ܵķ�Ӧ��2Cu+O2+2H2SO4��ϡ��
CuSO4+SO2��+2H2O���ӷ�Ӧ����ʽ���������������+6�۱�Ϊ�����е�+4��������ǿ�����ԣ��������ᷴӦ�������Σ���������������ԣ�;���ܵķ�Ӧ��2Cu+O2+2H2SO4��ϡ�� 2CuSO4+2H2O�ij����ӷ�ӦΪ2Cu+O2+4H+=2Cu2++2H2O��
2CuSO4+2H2O�ij����ӷ�ӦΪ2Cu+O2+4H+=2Cu2++2H2O���ʴ�Ϊ��ǿ����������ˣ� 2Cu+O2+4H+=2Cu2++2H2O��
��������1����������Ӧ����ƫ�����ƺ�������
��2���������ʵ����ʽ�Ͽ�ͼת����ϵ�����ò�Ʒ���н��
��3���������ױ������е��������������������ӣ�����������������KSCN��Һ��
��4���ӻ�����ԭ�ϵ������ʽǶȽ��
��5��ͭ��Ũ���ᷴӦ������Ũ�����ǿ�����Ժ����ԣ�ͭ���������ᷴӦ����ͭ���ӣ�
������������Ҫ�����������ӹ�ҵ��������ȡ�����ע��ӿ�ͼת�����ҳ�������Ϣ�������йصĻ�ѧ��Ӧ���н���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ