��Ŀ����
���ײ����ھ������ͬѧ���ﶼ�dz����ء�ij��ѧѧϰС�����ͨ�������й����ϣ��Լ�����ʵ�飬�����������ۡ���ʵ��ԭ����
(1)����������������������Һ�Ʊ�������������(�ܽ�Ƚ�С)���÷�Ӧ�Ļ�ѧ����ʽ��
__________________________________________________________________
(2)���ղ����������壺FeC2O4��2H2O![]() Fe+2CO2��+2H2O��
Fe+2CO2��+2H2O��
���������衿
(1)��������������Ʊ�
![]()
�������ձ�����������ҩ�ס���ֽ��������ƽ��FeSO4�������ʵ����Ʒ��Ϊ��������������Һ��ȱ�ٵ�������______________________________________________________________��
�������Ƶ�FeSO2��Һ�Գʻ�ɫ�����ܵ�ԭ����_____________________________________��
�ۼ���������������Ƿ�ϴ���ķ�����_____________________________________________��
(2)�������۵��Ʊ�
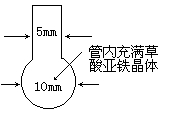
��һ������ԼΪ12 cm��14 cm�IJ������ھƾ���������Ƴ���ͼ��ʾ��״�������м����ɫ�����������塣�ٽ��ܿڲ��ּ�����ϸ��Ȼ���ھƾ�����Ͼ��ȼ��ȡ�������������ĩ������ȫ��ڣ��������ܿ���ϸ�IJ��ַ��ڻ������۷�գ�������õ��˸ߴ��ȵ��������ۡ�
��ʵ���зֽ����CO2�����������________________��
��ָ����ʦ����ͬѧ��Ӧȷ���ղ����ܵ����۷��ʱ�䡣����Ϊ���۹��硢�����ĺ����________________��

ʵ��ԭ��
FeSO4+H
��������
(1)��������������Ʊ�
��
����Ʒ�п��ܺ���Fe3+������ˮδ�����ȴ�������Һ����ʱ��ϳ�
��ȡ����ϴ��Һ���ⶨϴ��Һ��pH����ϴ��Һ��pH����7��˵���Ѿ�ϴ�Ӹɾ�
(2)�������۵��Ʊ�
������װ���п�������ֹ���ȵ���������
�ڹ�������������������������۴��Ȳ���(����δ����ȫ�ֽ⡢���������������)


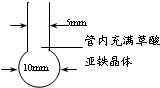 ������õ��˸ߴ��ȵ��������ۣ�
������õ��˸ߴ��ȵ��������ۣ� Fe+2CO2��+2H2O��
Fe+2CO2��+2H2O��