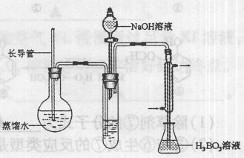
��Ŀ����
(18��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
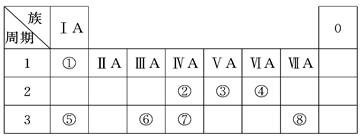
(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����________________��
(2)�ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
__________��ԭ�Ӱ뾶�����õ��������������ǽ�����������
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ_______________��
(4)�ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ��������(�����)________��
a��MnO2����b�� CuSO4 c��Na2SO3 d��FeCl3
(5) W��������ڵ�ͬ����Ԫ�ء����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
(6)�ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��

X��Һ��Y��Һ��Ӧ�����ӷ���ʽ______________��
N���ĵ��ʵĻ�ѧ����ʽΪ____________��
M��Һ������Ũ���ɴ�С������˳����______________ ��
M�������ӵļ������� __________��

(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����________________��
(2)�ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
__________��ԭ�Ӱ뾶�����õ��������������ǽ�����������
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ_______________��
(4)�ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ��������(�����)________��
a��MnO2����b�� CuSO4 c��Na2SO3 d��FeCl3
(5) W��������ڵ�ͬ����Ԫ�ء����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3��3H3PO3===3H3PO4��H2W�� |
| 1 | | |
| 2 | | |

X��Һ��Y��Һ��Ӧ�����ӷ���ʽ______________��
N���ĵ��ʵĻ�ѧ����ʽΪ____________��
M��Һ������Ũ���ɴ�С������˳����______________ ��
M�������ӵļ������� __________��
(18��)
��1���������ڣ���IVA�� -------------------------------------------------------------1��
��2��ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ�����������----------------------------1��
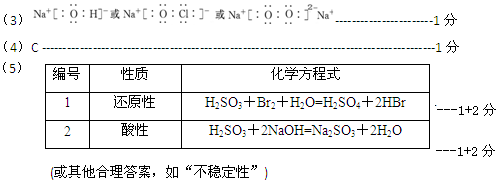
��6��Al3����3NH3��H2O===Al(OH)3����3NH4+ -------------------------------------------2��
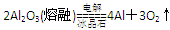 -------------------------------------------------------2��
-------------------------------------------------------2��
c(Cl-)>c(NH4+)>c(H+)>c(OH-)��c(NO3-)>c(NH4+)>c(H+)>c(OH-) ------------2��
ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠�����-------------------------------------------------2��
��1���������ڣ���IVA�� -------------------------------------------------------------1��
��2��ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ�����������----------------------------1��

��6��Al3����3NH3��H2O===Al(OH)3����3NH4+ -------------------------------------------2��
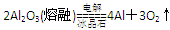 -------------------------------------------------------2��
-------------------------------------------------------2��c(Cl-)>c(NH4+)>c(H+)>c(OH-)��c(NO3-)>c(NH4+)>c(H+)>c(OH-) ------------2��
ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠�����-------------------------------------------------2��
�����ǿ���Ԫ�����ڱ���Ԫ�������ɵ���Ŀ����Ϊ�����Ƚ�ͼ��Ԫ����������ڱ���Ӧ������ȷ��ÿһ��Ԫ�ء���4���в������÷�Ӧ���������ʣ��������䣨H2O2��������Ӧ����ΪNa2SO3���л�ԭ�ԣ��ܷ�Ӧ����ѡC����5������H2SO3�����ʣ���Ϊ��Ԫ��ǿ�㣬�������ͨ�ԣ������ԡ���ԭ�Եȵȡ���6��ͻ�ƿ�ΪMΪ�����ǽ���Ԫ�ص��Σ���Ϊ��Σ�ZN���ޣ�Al������ZΪAl(OH)3��MΪ��Σ��б���Ԫ����ɣ�����ΪNH4Cl��NH4NO3��NH����ˮ�⣬����c(Cl-)>c(NH4+)>c(H+)>c(OH-)��c(NO3-)>c(NH4+)>c(H+)>c(OH-)��NH�ļ��鷽��Ϊ��������������Һ�����ȣ�����NH3���ɡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

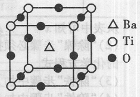
 ���ʵķ���ʽΪ ��
���ʵķ���ʽΪ ��
 ̬�̺����Լ�������
̬�̺����Լ�������