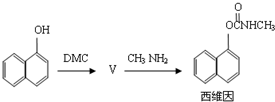
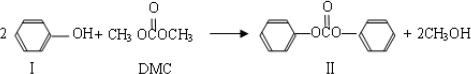
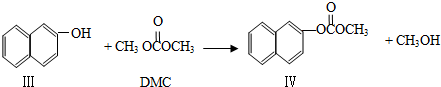
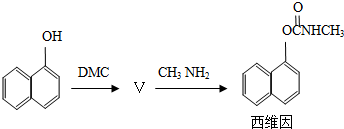
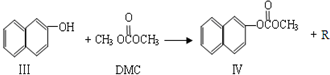
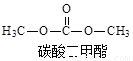��Ŀ����
̼�������(DMC)��һ�ֽ������ܵ��㷺��ע�Ļ�������ɫ������Ʒ���ڴ��������£����ɼ״���CO2ֱ�Ӻϳ�DMC��CO2 + 2CH3OH �� CO(OCH3)2 + H2O�����״�ת����ͨ�����ᳬ��1%����Լ�÷�Ӧ����ҵ������Ҫԭ��ij�о�С���������������������£�ͨ���о��¶ȡ���Ӧʱ�䡢���������ֱ��ת����(TON)��Ӱ�������۴����Ĵ�Ч�������㹫ʽΪ��TON=ת���ļ״������ʵ���/���������ʵ�����
��1����֪25��ʱ���״���DMC�ı�ȼ���ȷֱ�Ϊ��H1�͡�H2����������Ӧ��25��ʱ���ʱ��H3=_____��
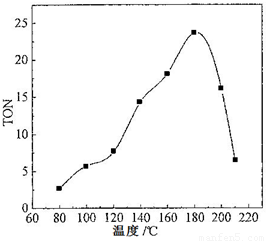
��2�����ݷ�Ӧ�¶ȶ�TON��Ӱ��ͼ������ͼ���жϸ÷�Ӧ���ʱ��H________0���>������=����<������������________________________________��
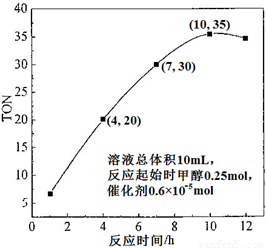
��3�����ݷ�Ӧʱ���TON��Ӱ��ͼ������ͼ������֪��Һ�����10mL����Ӧ��ʼʱ�״�0.25mol������0.6��10��5 mol��������¶��£�4��7 h��DMC��ƽ����Ӧ���ʣ�________������10 hʱ���״���ת���ʣ�________��
��4�����ݸ��о�С���ʵ�鼰����������TON��Ӱ��ͼ����ͼ�����ж�����˵����ȷ����___ __��
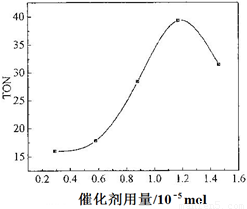
a. �ɼ״���CO2ֱ�Ӻϳ�DMC���������ü����õļ״���Ӱ�컷������������CO2ת��Ϊ��Դ������Դѭ�����úͻ����������涼������Ҫ����
b. �ڷ�Ӧ��ϵ�����Ӻ��ʵ���ˮ��������߸÷�Ӧ��TON
c. ��������������1.2��10��5 molʱ�����Ŵ������������ӣ��״���ƽ��ת�����������
d. ��������������1.2��10��5 molʱ�����Ŵ������������ӣ�DMC�IJ��ʷ��������½�
��15�֣�
��1��2��H1 �� ��H2 ��3�֣�
��2��< ��2�֣� ������ͬ�ķ�Ӧʱ�䣬�¶Ƚϵ�ʱ����Ӧδ�ﵽƽ�⣻�¶Ƚϸ�ʱ����Ӧ�Ѵﵽƽ�⣬�����¶����ߣ�TON��С����ƽ�������ƶ���˵���÷�Ӧ���ȣ�3�֣�
��3��1��10-3 mol��L-1��h-1 �� 2�֣� 8.4��10-2 % ��2�֣�
��4��ab��3�֣�
��������
���������
��1�����ݷ�Ӧ��CH3OH(l)+1.5O2(g)=CO2(g)+2H2O(l) ��H1����CO(OCH3)2(l)+3O2(g)=3CO2(g)+3H2O(l) ��H1�����ݸ�˹������Ŀ�귴Ӧ���ڢ١�2-�ڣ����Ц�H3=2��H1-��H2
��2������ͼ��֪���¶����ߣ�ƽ�������ƶ���������Ӧ���Ȧ�H<0��
��3��4h��TON=20��7h��TON=30����TON=10�����״�=10��0.6��10-5mol=0.6��10-4mol������Ϊ0.6��10-4mol��0.01L��3h=2��10-3 mol��L-1��h-1��10h��ת���ļ״�Ϊ35��0.6��10-5mol=2.1��10-4mol��ת����Ϊ2.1��10-4mol��0.25mol=8.4��10-2 %
��4��a����ȷ��b������ˮ��ʹƽ�������ƶ�����ȷ��cd��TON��ת���ʲ���һ��������
���㣺���黯ѧ��Ӧ���ʼ���ѧƽ������֪ʶ���漰����Ϣ�Ļ�ȡ�ͽ������������

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�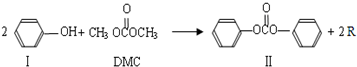
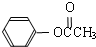 ���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����
���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����