��Ŀ����
ij�¶��£���һ���������н������·�Ӧ N2+3H2 2NH3���������һ����˵����Ӧ �Ѵﵽƽ����ǣ� ��
2NH3���������һ����˵����Ӧ �Ѵﵽƽ����ǣ� ��
��������ѹǿ����ʱ����仯
�ڵ�λʱ���ڣ��� 3molH2 ��Ӧ��ͬʱ�� 2molNH3 ����
��������ܶȲ���ʱ����仯
�ܵ�λʱ���ڣ��� 1molN2 ���ɣ�ͬʱ�� 2molNH3 ����
���� N2�� H2��NH3 ��ʾ�ĸ÷�Ӧ�Ļ�ѧ��Ӧ����֮��Ϊ 1��3��2
�������ƽ��Ħ����������ʱ����仯
A���٢ܢ� B���٢ڢ� C�� �ڢۢ� D���٢ڢ�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�ijʵ��С����������к͵ζ����ⶨʳ����������g/100mL��������������뱾ʵ�鲢�ش�������⡣���й�ʵ��ҩƷΪ������ʳ�ð״���Ʒ500mL��0.1000mol/LNaOH����Һ������ˮ��0.1%������Һ��0.1%��̪��Һ��0.1%ʯ����Һ����
ʵ�鲽�裺
A���õζ�����ȡ10mL���۰״���Ʒ������100mL����ƿ�У�������ˮ����г�ȥCO2��Ѹ����ȴ��ϡ�����̶��ߣ�ҡ�ȼ��ô���ʳ����Һ��
B������ʽ�ζ���ȡ����ʳ����Һ20.00mL����ƿ�У����μӷ�ָ̪ʾ�����á�
C����ʽ�ζ���ʢװ��NaOH��Һ�����ú�ȡ���ݣ���¼ΪNaOH����Һ����ij�������
D���ζ�������¼NaOH���ն������ظ��ζ�2-3�Ρ�
E��ʵ�����ݼ�¼
1 | 2 | 3 | 4 | |
V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
��1����c����Ʒ��/moL•L-1=_________����Ʒ������=_________g/100mL��
��2���ж�D�������ʱ��Һ����ζ��յ�ķ�����________________��
��3��������B�������֮ǰ�����ô���Һ��ϴ��ƿ����Եζ����������Ӱ����_________(���Ӱ�족����ƫ��ƫС������ͬ)������D�����ü�ʽ�ζ���ʱ��ʼû�����ݣ���������ݣ���Եζ����������Ӱ����___________����D����ζ�ǰƽ�Ӷ������ζ��յ�ʱ���Ӷ��� ����Եζ����������Ӱ����__________��
����Եζ����������Ӱ����__________��
ú��һ����Ҫ�Ļ���ԭ�ϣ����ǽ�����ú��ȡ��ˮú������̿�����ѵȹ㷺���ڹ�ũҵ�����С�(1)��֪:
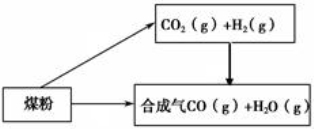
��C(s) �� H2O(g) = CO(g)��H2(g) ��H����131.3 kJ��mol ��1
��1
��CO2(g) �� H2(g) = CO(g) �� H2O(g) ��H��+41.3 kJ��mol��1
��̼��ˮ������Ӧ�� �ɶ�����̼���������Ȼ�ѧ����ʽΪ
�ɶ�����̼���������Ȼ�ѧ����ʽΪ
�÷�Ӧ�� (����¡��������¡����κ��¶ȡ�)�������������Է����С�
(2)��������̿��ԭ�������������������ӦC(s)+2NO(g) N2(g)+CO2(g)����ij�ܱ������м���һ�����Ļ���̿��NO����T1��ʱ����ͬʱ���ø����ʵ�Ũ�����±���ʾ��
N2(g)+CO2(g)����ij�ܱ������м���һ�����Ļ���̿��NO����T1��ʱ����ͬʱ���ø����ʵ�Ũ�����±���ʾ��
ʱ��(min) Ũ��(mol��L-1) | 0 | 10 | 20 | 30 | 40 | 50 (3)�о���������ӦCO(g)+H2O(g)
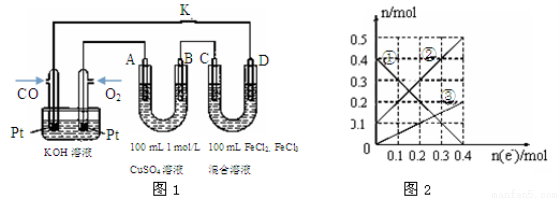
����Ӧ��500��ʱ���У�����ʼʱCO��H2O��Ũ�Ⱦ�Ϊ0.020 mol��L-1���ڸ������´ﵽƽ��ʱ��CO��ת����Ϊ (4)��CO��ȼ�ϵ�ص��CuSO4��Һ��FeCl3��FeCl2���Һ��ʾ��ͼ��ͼ1��ʾ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��
ͼ1��A�������������ڱ�״���µ����Ϊ �ڵ缫ΪC��D��װ����Һ�н��������ӵ����ʵ�����ת�Ƶ��ӵ����ʵ����仯��ϵ��ͼ2��ʾ����ͼ�Т��߱�ʾ���� (�����ӷ���)�ı仯����Ӧ������Ҫʹ��װ���н���������ǡ����ȫ��������Ҫ mL 5.0 mol��L��1NaOH��Һ�� |



 )��Ӧ����NH4Cl��s��
)��Ӧ����NH4Cl��s��

 Ni(CO)4(g)����֪�÷�Ӧ��25�桢80��ʱ��ƽ�ⳣ���ֱ�
Ni(CO)4(g)����֪�÷�Ӧ��25�桢80��ʱ��ƽ�ⳣ���ֱ� Ϊ5��104��2������˵����ȷ����( )
Ϊ5��104��2������˵����ȷ����( )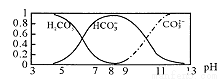

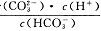 ����
����
 H2(g)+CO2(g)ƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2(g)+CO2(g)ƽ�ⳣ�����¶ȵı仯���±���ʾ��