��Ŀ����
����Ŀ������ѧ-���ʽṹ�����ʡ�
��λ������Ϊ����ɫ���壬��ԭ���������������A��B��C��D��E����Ԫ����ɣ���ԭ�Ӹ�����Ϊl4:4:5:1:1������C��DԪ��ͬ������ԭ������DΪC�Ķ�����EԪ�ص���Χ�����Ų�Ϊ(n-1)dn+6nsl���ش��������⡣
��1��д��Ԫ��D�����ڱ��е�λ���� ��Bԭ�ӵ���Χ�����Ų�ͼ ��Eԭ�ӵĺ����� �ֲ�ͬ�˶�״̬�ĵ��ӣ�B��C�ĵ�һ�����ܴ�С��ϵ ��(��Ԫ�ط��ű�ʾ)
��2��CԪ�ؿ���AԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1:1��l:2������ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽ �����ֻ����������Ȼ��ܣ���������Ҫԭ��Ϊ ��
��3������λ������Ļ�ѧʽΪ ��
��4��AԪ����BԪ�ؿ��γɷ���ʽΪA2B2��ij������û�����ķ��Ӿ���ƽ��ṹ������ṹʽΪ ��
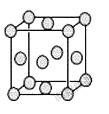
��5����֪E�ľ����ṹ��ͼ��ʾ���˾���������ı߳�Ϊa cm��E���ʵ��ܶ�Ϊpg.cm-3����٤������Ϊ ����a��p��ʾ�����þ�����λ��Ϊ ��EDC4�������Һ������DC![]() �Ŀռ乹���� ������Dԭ�ӵ��ӻ���������� �������EDC4��ˮ��Һ�������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
�Ŀռ乹���� ������Dԭ�ӵ��ӻ���������� �������EDC4��ˮ��Һ�������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
���𰸡�
��1���� VIA ![]() 29 N>O
29 N>O
��2�� ![]() H2O��H2O2֮���γ����
H2O��H2O2֮���γ����
��3��Cu(NH3)4SO4H2O ��4��N-N=N-H
��5��NA=256/p.a3 12 ��������
sp3�ӻ� 2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4
2Cu+O2��+2H2SO4
��������
���������EԪ�ص���Χ�����Ų�Ϊ(n-1)dn��6nsl����Ϊs���û������������ݺ��ع����֪����ʱd���Ӧ����ȫ����״̬����n��6��10�����n��4������EԪ�ص�ԭ��������18��10��1��29����E��ͭԪ�ء���λ������Ϊ����ɫ���壬��ԭ���������������A��B��C��D��E����Ԫ����ɣ���ԭ�Ӹ�����Ϊl4:4:5:1:1������C��DԪ��ͬ������ԭ������DΪC�Ķ���������������Ԫ������Ԫ������Ԫ�أ���C��O��D��S�����ݸ���λ��������ԭ�Ӹ����ȿ�֪��A����Ԫ�أ�S��O��������������ʣ��1����ԭ����2����ԭ�ӽ���γ�1����ˮ����4��Bԭ�ӽ��12����ԭ�ӣ�������֮��Ϊ1:3�����Ը������ǰ�������B�ǵ�Ԫ�ء�
��1�����������ƶϣ�DΪ��Ԫ�أ���Ԫ�������ڱ��е�λ���ǵ������ڵ���A�壻BΪ��Ԫ�أ�Nԭ�ӵ���Χ�����Ų�ͼΪ![]() ��EΪ29��Ԫ��ͭ��ԭ�ӵĺ�����29�ֲ�ͬ�˶�״̬�ĵ��ӣ�B��C�ֱ�ΪN��O��Nԭ�ӵ������p�����3�����ӣ����ڰ����״̬���Ƚ��ȶ�����һ�����ܱ�����Ԫ�صĵ�һ�����ܴ����һ�����ܵĴ�С��ϵΪN>O��
��EΪ29��Ԫ��ͭ��ԭ�ӵĺ�����29�ֲ�ͬ�˶�״̬�ĵ��ӣ�B��C�ֱ�ΪN��O��Nԭ�ӵ������p�����3�����ӣ����ڰ����״̬���Ƚ��ȶ�����һ�����ܱ�����Ԫ�صĵ�һ�����ܴ����һ�����ܵĴ�С��ϵΪN>O��
��2��C��A�ֱ�ΪO��H��OԪ�ؿ���HԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1:1��l:2���û�����ΪH2O��H2O2 ��ԭ�Ӹ�����Ϊ1:1�Ļ�����H2O2�ĵ���ʽΪ![]() ��H2O��H2O2֮���γ�����������ֻ����������Ȼ��ܡ�
��H2O��H2O2֮���γ�����������ֻ����������Ȼ��ܡ�
��3������λ�������а�������������ѧʽΪCu(NH3)4SO4H2O ��
��4����Ԫ���뵪Ԫ�ؿ��γɷ���ʽΪA2B2�Ļ�������N2H2���û�����ķ��Ӿ���ƽ��ṹ������8�����ȶ��ṹ��֪����Ԫ���뵪Ԫ��֮���γ�˫������Ԫ���뵪Ԫ��֮���γɵ�������ṹʽΪH-N��N-H��
��5�����ݾ����ṹ��֪�������к���ͭԭ�ӵĸ�����8��1/8��6��1/2��4�����p��a3��NA��4��64�����NA=256/p.a3���þ�����λ��Ϊ3��8��2=12�����������������ԭ�Ӳ����ڹ¶Ե��ӣ���۲���Ӷ�����4�����Կռ乹�������������Σ���ԭ����sp3�ӻ����������ͭ��Һ����ͭ�����������ᣬ������ܷ�Ӧ�Ļ�ѧ����ʽΪ2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4��
2Cu+O2��+2H2SO4��


