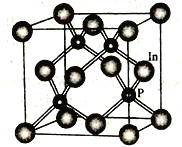
题目内容
【题目】过氧化钙是一种新型的多功能无机化工产品,常温下是无色或淡黄色粉末,易溶于酸,难溶于水、乙醇等溶剂。某实验小组在低温和通入氨气的碱性条件下,利用钙盐与过氧化氢反应制取CaO2·8H2O沉淀(该反应是一个放热反应)。
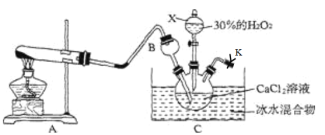
(1)仪器X的名称是___,检查整个装置气密性的方法为:____
(2)仪器B的作用是_______,CaO2的电子式为______
(3)写出A中发生反应的化学方程式:______。
(4)制取CaO2·8H2O时,冰水浴维持反应在0~5℃的低温下进行,原因是____。生成CaO2·8H2O的化学方程为____。
(5)2.76 gCaO2·8H2O样品受热脱水过程的热重曲线(样品质量随温度变化的曲线,140℃时完全脱水,杂质受热不分解)如图所示。
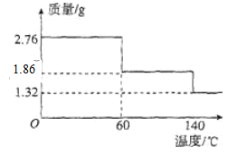
①试确定60℃时CaO2·xH2O中x=______。
②该样品中CaO2的质量分数为______(保留4位有效数字)。
【答案】分液漏斗 将K关闭,从分液漏斗中加水,一段时间后,液体不能顺利滴下 ![]() 防止倒吸 2NH4Cl+Ca(OH)2
防止倒吸 2NH4Cl+Ca(OH)2![]() CaCl2+2NH3↑+2H2O 低于0℃,液体易冻结,反应困难,温度过高,过氧化氢分解速率加快 CaCl2+2NH3+8H2O+2H2O2=CaO2·8H2O+2NH4Cl 3 26.09‰
CaCl2+2NH3↑+2H2O 低于0℃,液体易冻结,反应困难,温度过高,过氧化氢分解速率加快 CaCl2+2NH3+8H2O+2H2O2=CaO2·8H2O+2NH4Cl 3 26.09‰
【解析】
(1)根据X的特征可知为分液漏斗;检查整个装置气密性的方法为将K关闭,从分液漏斗中加水,一段时间后,液体不能顺利滴下,说明气密性良好;
(2)氨气极易溶于水,长颈漏斗插入三颈烧瓶的溶液中防止倒吸;CaO2为离子化合物,由钙离子和过氧根离子组成;
(3)实验室中用氯化铵和氢氧化钙固体共热制取氨气;
(4)低于O℃,液体易冻结,反应困难,温度过高,过氧化氢分解速率加快,CaCl2与H2O2、NH3、H2O反应生成CaO28H2O与NH4Cl;
(5)①140℃时完全脱水,杂质受热不分解,结合样品总质量计算出结晶水的总质量、物质的量,从而得出样品中CaO28H2O物质的量,再根据60℃时固体的质量计算出失去结晶水的质量、物质的量,进而计算出CaO2xH2O中的x;
②根据①计算出的样品中CaO2的物质的量,根据m=nM计算出其质量,再计算出样品中CaO28H2O的纯度
(1)根据X的构造可知X为分液漏斗;检查整个装置气密性的方法为将K关闭,从分液漏斗中加水,一段时间后,液体不能顺利滴下,说明气密性良好,故答案为:分液漏斗;将K关闭,从分液漏斗中加水,一段时间后,液体不能顺利滴下;
(2)B为长颈漏斗,插入三颈烧瓶的溶液中,可以防止因氨气极易溶于水而产生倒吸;CaO2为离子化合物,由钙离子和过氧根离子组成,电子式为![]() ,故答案为:防倒吸;
,故答案为:防倒吸;![]() ;
;
(3)实验室中用氯化铵和氢氧化钙固体共热制取氨气,反应的化学方程式为2NH4Cl+Ca(OH)2![]() CaCl2+2NH3↑+2H2O,故答案为:2NH4Cl+Ca(OH)2
CaCl2+2NH3↑+2H2O,故答案为:2NH4Cl+Ca(OH)2![]() CaCl2+2NH3↑+2H2O;
CaCl2+2NH3↑+2H2O;
(4)由于低于0℃,液体易冻结,反应困难,而温度较高,过氧化氢分解速率加快,所以制取CaO28H2O一般在0℃~5℃的低温下进行;根据装置C中的物质得出制取的原理方程式为:CaCl2+H2O2+2NH3+8H2O=2NH4Cl+CaO28H2O,故答案为:低于0℃,液体易冻结,反应困难,温度较高,过氧化氢分解速率加快;CaCl2+H2O2+2NH3+8H2O=2NH4Cl+CaO28H2O;(5)①140℃时完全脱水,杂质受热不分解,则样品中CaO28H2O含有的结晶水的总质量为(2.76g-1.32g)=1.44g,结晶水的物质的量为![]() =0.08mol,60℃时固体的质量为1.86g,失去结晶水的质量为:2.76g-1. 86g=0.9g,失去结晶水的物质的量为
=0.08mol,60℃时固体的质量为1.86g,失去结晶水的质量为:2.76g-1. 86g=0.9g,失去结晶水的物质的量为![]() =0.05mol,原样品中含有CaO28H2O的物质的量为
=0.05mol,原样品中含有CaO28H2O的物质的量为![]() =0.01mol,所以60℃时失去结晶水的个数为
=0.01mol,所以60℃时失去结晶水的个数为![]() =5,故60℃时CaO2xH2O中x=8-,5=3,故答案为:3;
=5,故60℃时CaO2xH2O中x=8-,5=3,故答案为:3;
②根据①可知CaO28H2O的物质的量为0.01mol,则CaO2的物质的量也是0.01mol,其质量为:72g/mol×0.01mol=0.72g,样品中CaO2的纯度为![]() ×100%≈26.09%,故答案为:26.09%。
×100%≈26.09%,故答案为:26.09%。

【题目】昆明得天独厚的气候地理条件,造就了亚洲第一鲜花交易市场。生活中人们为了延长鲜花的寿命,通常会加入鲜花保鲜剂。
下表是0.25 L某种鲜花保鲜剂中含有的成分及含量(部分成分省略)。回答下列问题:
成分 | 质量(g) | 摩尔质量(g/mol) |
蔗糖(C12H22O11) | 12.50 | 342 |
硫酸钾(K2SO4) | 0.125 | 174 |
高锰酸钾(KMnO4) | 0.125 | 158 |
硝酸银(AgNO3) | 0.01 | 170 |
…… | …… | …… |
(1)鲜花保鲜剂的下列成分中,不属于电解质的是__________(填标号)。
a.C12H22O11 b.K2SO4 c.KMnO4 d.AgNO3
(2)欲配制250 mL该鲜花保鲜剂,现已提供下列仪器:①250mL容量瓶②量筒③烧杯④药匙⑤电子天平,如要完成实验,缺少的玻璃仪器还有_________、_________ (写仪器名称)。
(3)下列操作会使所配鲜花保鲜剂浓度偏低的是__________(填标号)。
a.容量瓶用蒸馏水洗净后没有烘干 b.用玻璃棒引流,将溶液转移到容量瓶中时有溶液洒到了容量瓶外面 c.定容时俯视刻度线 d.滴加蒸馏水,使溶液凹面刚好与刻度线相切,盖上瓶塞反复摇匀后,静置,发现液面比刻度线低,再加水至刻度线
(4)写出该鲜花保鲜剂中K+的物质的量浓度的计算式(省略成分中不含K+)_______(不必化简)。