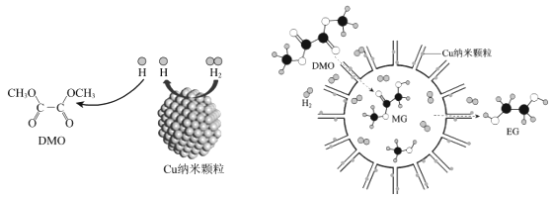
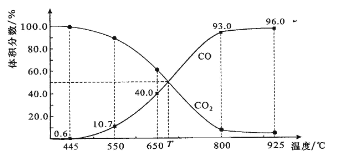
��Ŀ����
����Ŀ���������[Ca��NO2��2]��ˮ���������Ӽ�����Ҫԭ�ϣ�ijѧϰС�����ʵ���Ʊ�������ƣ�ʵ��װ����ͼ��ʾ���г�װ����ȥ����

��֪��2NO��CaO2��Ca��NO2��2��2NO2��CaO2��Ca��NO3��2��
��ش��������⣺
��1����������ƿ�м���ϡ����֮ǰ��Ӧ��װ����ͨ��һ��ʱ���N2��ԭ��Ϊ���÷���ʽ��ʾ��_________��
��2��װ��B�����Լ���__________�������dz�ȥ__________���ѧʽ����
��3��װ��E�У�����K2Cr2O7��Һ�ɽ�ʣ���NO������![]() ����Һ�ɳ�ɫ��Ϊ��ɫ��Cr3������������Ӧ�����ӷ���ʽ��__________��
����Һ�ɳ�ɫ��Ϊ��ɫ��Cr3������������Ӧ�����ӷ���ʽ��__________��
��4����֪��Ca��NO2��2��Һ��������NO���塣���ʵ��֤��װ��D��������������ɣ�_________��
��5����ҵ�Ͽ���ʯ��������Ṥҵ��β������NO��NO2����Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca��NO2��2����Ӧԭ��ΪCa��OH��2��NO��NO2��Ca��NO2��2��H2O��
����n��NO�� ��n��NO2����1 ��1����ᵼ��_______________��
����n��NO�� ��n��NO2����1 ��1����ᵼ��________________��
���𰸡�2NO+O2=2NO2 ����ˮ������������Һ������������ HNO3 Cr2O72-��2NO+6H+= 2Cr3++2NO3-��3H2O ȡ����װ��D�з�Ӧ��Ĺ����ڽྻ���Թ��У��μ�����ϡ���ᣬ�Թܿ��к���ɫ������� �ŷ�NO���壬��Ⱦ���� ��Ʒ�л���Ca(NO3)2����
��������
(1)��װ���к��п���������������������һ������������ͨ�뵪����Ŀ�����ų�װ������������ֹ�佫���ɵ�һ�������������ɶ���������
(2)�������ӷ���
(3)����Ӧ��Cr2O72-����ԭ��Cr3+��NO����ΪNO3-�����ݵ��ӵ�ʧ�غ��������غ���ƽ��
(4)������Ca(NO3)2����ᷢ����Ӧ����NO���壬NO�������������ɺ���ɫNO2���壻
(5)������n(NO):n(NO2)>1:1,��һ����������������<1: 1�����������������
(1)��װ���к��п���������������������һ������������ͨ�뵪����Ŀ�����ų�װ������������ֹ�佫���ɵ�һ�������������ɶ���������
�ʴ�Ϊ��2NO+O2=2NO2��
(2)�������ӷ���ͨ��B��ʢ�ŵ�����ˮ������������Һϴȥ���ᣬ�Է�ֹ�������Ʒ�Ӧ����������
�ʴ�Ϊ������ˮ������������Һ�����������𰸣�HNO3��
(3)����Ӧ��K2Cr2O7����ԭ��Cr3+��NO����ΪNO3-�����ӷ�Ӧ����ʽΪ��Cr2O72-��2NO+6H+= 2Cr3++2NO3-��3H2O��
�ʴ�Ϊ��Cr2O72-��2NO+6H+= 2Cr3++2NO3-��3H2O��
(4)��Ca(NO3)2��Һ��������NO���壬NO�������������ɺ���ɫNO2���壬����ȡ����E�з�Ӧ��Ĺ������Թ��У��μ��������ᣬ�Թܿ��к���ɫ�������ɼ���֤��E��������������ɣ�
�ʴ�Ϊ��ȡ����װ��E�з�Ӧ��Ĺ����ڽྻ���Թ��У��μ�����ϡ���ᣬ�Թܿ��к���ɫ�����������װ��E��������������ɣ�
(5)�١���n(NO):n(NO2)>l:l����һ�������������ŷ�������NO�������ߣ���Ⱦ������
�ڡ���n(NO):n(NO2)<l:l�����������������������������ʯ���鷴Ӧ����Ca(NO3)2��
�ʴ�Ϊ���ŷ�NO���壬��Ⱦ��������Ʒ�л���Ca(NO3)2���ʡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������仯������NH3����Ρ�N2H4��N2O4������ѧ��ѧ��������ҵ������������ռ����Ҫ��λ��
(1)���亽����������(N2H4)��N2O4��ȼ������ȼ������(N2H4)��N2O4�ķ�ӦΪ
2N2H4 (g)+ N2O4(g)==3N2(g)+4H2O(g) ��H=-1077 kJ��mol-1��
��֪��ط�Ӧ�Ļ�ѧ�������������±���ʾ��
��ѧ�� | N��H | N��N |
| O��H |
E/(kJ��mol��1) | 390 | 190 | 946 | 460 |
��ʹ1 mol N2O4(g)�����л�ѧ����ȫ����ʱ��Ҫ���յ�������________________��
��������˵��2N2H4 (g)+ N2O4(g)==3N2(g)+4H2O(g) ��H ��ƽ��״̬����________
a.��������ƽ����Է����������� b.V��N2��=3V�� N2O4��
c.N2H4���������ֲ��� d. ��H���ٱ仯
(2)N2O4��NO2֮����ڷ�ӦN2O4(g) ![]() 2NO2(g)����һ������N2O4���˺����ܱ������У������ƽ��ת����[��(N2O4)]���¶ȵı仯����ͼ��ʾ��
2NO2(g)����һ������N2O4���˺����ܱ������У������ƽ��ת����[��(N2O4)]���¶ȵı仯����ͼ��ʾ��
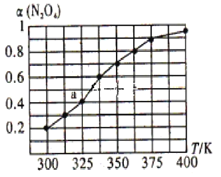
����ͼ�Ʋ�÷�Ӧ�ġ�H_______0(��>����<��)������Ϊ____________________________��
��ͼ��a���Ӧ�¶��£���֪N2O4����ʼѹǿp0Ϊ108 kPa������¶��·�Ӧ��ƽ�ⳣ��Kp��________________ (��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
(3)���NO2�Ʊ�NH4NO3���乤��ԭ������ͼ��ʾ��
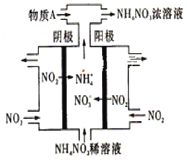
�������ĵ缫��ӦʽΪ____________________________________________________��
��Ϊʹ������ȫ��ת��ΪNH4NO3���貹��ij�ֻ����������A����A�Ļ�ѧʽΪ________________��