��Ŀ����
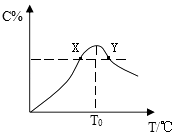
����Ŀ�������£���Ũ�Ⱦ�Ϊ0.1 mol��L��1�������Ϊ100 mL������һԪ��HX��HY����Һ�У��ֱ����NaOH���壬lg![]() �����NaOH�����ʵ����ı仯��ͼ��ʾ(���Լ���NaOH���嵼����Һ�¶�������ı仯)������������ȷ����
�����NaOH�����ʵ����ı仯��ͼ��ʾ(���Լ���NaOH���嵼����Һ�¶�������ı仯)������������ȷ����

A. a����ˮ�������c(H��)��10��12 mol��L��1
B. b��ʱ���ǡ����ȫ�к�
C. c����Һ�У�c(Y��)>c(HY)
D. HX��HY������������HX>HY
���𰸡�C
��������A��a��lg  =12������Һ��c(H+)=0.1mol/L����Һ��ˮ�����c(H+)=
=12������Һ��c(H+)=0.1mol/L����Һ��ˮ�����c(H+)=![]() =10-13molL-1����A����B��Ũ�Ⱦ�Ϊ0.1molL-1�������100mL��HY��NaOHǡ���к�����NaOHΪ0.01mol����b��ʱ���ĵ�NaOHΪ0.008mol���������������B����C��c��lg
=10-13molL-1����A����B��Ũ�Ⱦ�Ϊ0.1molL-1�������100mL��HY��NaOHǡ���к�����NaOHΪ0.01mol����b��ʱ���ĵ�NaOHΪ0.008mol���������������B����C��c��lg  =6������Һ��c(H+)=10-4mol/L����ʱ���ĵ�NaOHΪ0.005mol������Һ�е�����ΪNaY��HY��������Һ�����ԣ�����HY�ĵ���̶ȴ���NaY��ˮ��̶ȣ�����c(Y-)��c(HY)����C��ȷ��D��lg
=6������Һ��c(H+)=10-4mol/L����ʱ���ĵ�NaOHΪ0.005mol������Һ�е�����ΪNaY��HY��������Һ�����ԣ�����HY�ĵ���̶ȴ���NaY��ˮ��̶ȣ�����c(Y-)��c(HY)����C��ȷ��D��lg Խ����Һ��������Ũ��Խ��δ��NaOHʱ��HX��Һ��lg
Խ����Һ��������Ũ��Խ��δ��NaOHʱ��HX��Һ��lg  ��ֵ������HX�����Դ���HY��a��lg
��ֵ������HX�����Դ���HY��a��lg  =12����HA��Һ��c(H+)=0.1mol/L��HAΪǿ�ᣬ��D����ѡC��
=12����HA��Һ��c(H+)=0.1mol/L��HAΪǿ�ᣬ��D����ѡC��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣�������ŷֱ����ijһԪ�ء���ش��������⣮
���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | �� |
��1�������뵼����ϵ�Ԫ���� ______����Ԫ�ط��ţ���
��2����̬�⻯����������������ˮ�����ֱ�ӻ�������һ���ε�Ԫ���� ______�������ƣ���
��3���������������Ӱ뾶��С����___________�������ӷ��ţ���
��4���� ~ �������������ˮ�����У�������ǿ���� __________���ѧʽ����������ǿ����________���ѧʽ�������������������ˮ���ﷴӦ�����ӷ���ʽΪ__________��
��5���ࡢ����γ�A2B2�͵Ĺ��ۻ���������ж��߾�����8�����ȶ��ṹ���������ʽΪ_________________��