��Ŀ����
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ����ɫ |
| �ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ���� |
| ��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ�����V�������ɵij��������������壨n���Ĺ�ϵ��ͼ |
��1������ʵ�����жϸ������п϶������ڵ�������
��2��д����Һ�еμӵ���KI��Һʱ������Ӧ�����ӷ���ʽ��
��3����������Һ�μ�NaOH��Һ�����������������ж����Ӧ��д������������Ӧ�����ӷ���ʽ
| ||
| ||
��4�����ʵ�鷽������������Ƿ����Cl-��
��5����С��Ϊ��̽��NO����������������γɹ��̣�����ƿ�г��뺬������NO��SO2���壬������ͨ��O2��������ѧ��Ӧ����������������ˮ�������������꣬��NO��������Ӧ�е�������
��2�����ݵ��ʵ���ʹ����KI��Һ����ɫ��������
��3�����������Ժ������ӷ����кͷ�Ӧ�����Ժ������ӷ�����Ӧ�����Ժ�笠����ӷ�����Ӧ��
��4�����������ӵļ��鷽�����������ữ����������������ɫ��������Ҫ�ų�������ĸ��ţ�
��5���������ʵ���������д����ʽ������һ�������ڷ�Ӧ�е��ص����ж��������ã�
�ʴ�Ϊ��SO32-��Ba2+��Na+��Cl-��
��2���������������ӵ����ӷ���ʽΪ��6I-+2NO3-+8H+=3I2+2NO��+4H2O���ʴ�Ϊ��6I-+2NO3-+8H+=3I2+2NO��+4H2O��
��3����Һ�μ�NaOH��Һ���ܺ������ӷ����кͷ�Ӧ���ܺ������ӡ�笠����ӷ�����Ӧ����Ӧ����ʽΪ��OH-+H+=H2O��NH4++OH-
| ||
| ||
��4�������ӵļ��鷽�����������ữ����������������ɫ��������Ҫ�ų�������ĸ��ţ�
�ʴ�Ϊ��ȡ������Һ���μ�������Ba��NO3��2��Һ�����ã�ȡ�ϲ���Һ���μ������ữ��AgNO3��Һ�����а�ɫ���������������Cl-��
��5��һ���������л�ԭ�ԣ������ױ���������������ʽΪ��2NO+O2=2NO2�����������ܽ�������������������ʽΪNO2+SO2=SO3+NO����������һ������������û�б仯�����ڷ�Ӧ�����������ʴ�Ϊ��������

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�ѡ�� | ���� | ��Ⱦָ�� | ��Ҫ��Ⱦ�� | �������� |
A | ���� | 92 | TSP(������������) | �� |
B | ���� | 42 | �� | �� |
C | �麣 | 22 | �� | �� |
D | ���� | 98 | SO2 | �� |
�������������������γ��������Ҫ���ʡ�ij�������п��ܺ����������ӣ�Na����Ba2����NH4+��Al3����Cl����SO32-��SO42-��NO3-�ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
���� | �����Լ� | ʵ������ |
��һ����Һ | �μ������ĵ���?KI��Һ | ��Һ����ɫ |
�ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ�������� |
��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ���(V)�����ɵij�������������������ʵ���(n)�Ĺ�ϵ����ͼ |
|
��ش��������⣺
(1)����ʵ�����жϸ������п϶������ڵ�������______________������ȷ����������________________��
(2)д����һ����Һ�μӵ���?KI��Һʱ������Ӧ�����ӷ���ʽ��__________________��
(3)��������Һ�μ�NaOH��Һ�����ȣ����������з����˶����Ӧ��д������������Ӧ�����ӷ���ʽ��__________________________________��__________________________��
(4)���ʵ�鷽����������������Ƿ����Cl����___________________________________
______________________________��
(5)��С��Ϊ��̽��NO����������������γɹ��̣�����ƿ�г��뺬������NO��SO2���壬������ͨ��O2��������ѧ��Ӧ����������������ˮ�������������꣬��NO��������Ӧ�е�������________________________________________________________��
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ����ɫ |
| �ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ���� |
| ��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ�����V�������ɵij��������������壨n���Ĺ�ϵ��ͼ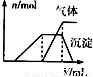 |
��1������ʵ�����жϸ������п϶������ڵ�������______������ȷ����������______��
��2��д����Һ�еμӵ���KI��Һʱ������Ӧ�����ӷ���ʽ��______��
��3����������Һ�μ�NaOH��Һ�����������������ж����Ӧ��д������������Ӧ�����ӷ���ʽ______ NH3��+H2O

