��Ŀ����
ijͬѧ�ú��������������ʵĹ�ҵ����ͭ��ȡ����������ͭ��̽��������
��1���Ʊ�����ͭ
�� ����ҵ����ͭ������ˮ�ܽ⡢���衢���˳�ȥ���������ʡ�����������Һ�к���Fe2+�IJ���������____________________________��
�� ����Һ�еμ�H2O2��Һ���Լ��ȣ���Fe2+ת����ȫ��Ϊ��Fe3+ȫ��ת��ΪFe(OH)3�����������Ὣ
Cu2+ת��Ϊ����������������Cu2(OH)2CO3��ĩ�����裬�Կ�����ҺpH=3.5��������к���ˣ���ϡ�����ữ��Һ��pH=1���ٴ���Һ�з��������ͭ���塣Fe2+ת��ΪFe3+�����ӷ���ʽ��______________��
�� �ڲⶨ��������ͭ���壨CuSO4��xH2O��xֵ��ʵ���У����õIJ�����������Ϊ���ƾ��ơ���������_______________��ʵ������г����������ٽ���_______�Ρ�
�� �õõ�������ͭ����������ͭ��
��2��̽������ͭ������
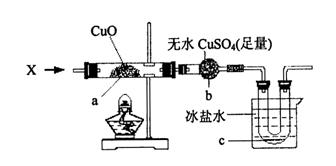
�ڼ��������£�����ͼ��ʾװ���г���ͨ��ij������X��������a����ɫ���ʱ�Ϊ��ɫ�� b����ɫ��ĩ��Ϊ��ɫ��c������ɫҺ����������������Ӧ��������ȫ��
��1���Ʊ�����ͭ
�� ����ҵ����ͭ������ˮ�ܽ⡢���衢���˳�ȥ���������ʡ�����������Һ�к���Fe2+�IJ���������____________________________��
�� ����Һ�еμ�H2O2��Һ���Լ��ȣ���Fe2+ת����ȫ��Ϊ��Fe3+ȫ��ת��ΪFe(OH)3�����������Ὣ
Cu2+ת��Ϊ����������������Cu2(OH)2CO3��ĩ�����裬�Կ�����ҺpH=3.5��������к���ˣ���ϡ�����ữ��Һ��pH=1���ٴ���Һ�з��������ͭ���塣Fe2+ת��ΪFe3+�����ӷ���ʽ��______________��
�� �ڲⶨ��������ͭ���壨CuSO4��xH2O��xֵ��ʵ���У����õIJ�����������Ϊ���ƾ��ơ���������_______________��ʵ������г����������ٽ���_______�Ρ�
�� �õõ�������ͭ����������ͭ��
��2��̽������ͭ������
�ڼ��������£�����ͼ��ʾװ���г���ͨ��ij������X��������a����ɫ���ʱ�Ϊ��ɫ�� b����ɫ��ĩ��Ϊ��ɫ��c������ɫҺ����������������Ӧ��������ȫ��

��X������____________����һ����ѧʽ���ɣ���X��CuO��Ӧ�Ļ�ѧ����ʽ��_____________��
��1����ȡ������Һ���Թ��У��μ�����KSCN��Һ�������ټ����������Ƶ���ˮ����Һ�ʺ�ɫ����֤����Һ�к���Fe2+���ӡ�
��2Fe2++H2O2+2H+==2Fe3++2H2O
�۸����� �� 4��
��2��CH3CH2OH ��CH3CH2OH+CuO CH3CHO+H2O+Cu
CH3CHO+H2O+Cu
��2Fe2++H2O2+2H+==2Fe3++2H2O
�۸����� �� 4��
��2��CH3CH2OH ��CH3CH2OH+CuO
 CH3CHO+H2O+Cu
CH3CHO+H2O+Cu
��ϰ��ϵ�д�
 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
�����Ŀ